
|
|
Lecture notes, cheat sheets
Anesthesiology and resuscitation. Lecture notes: briefly, the most important
Directory / Lecture notes, cheat sheets Table of contents
Lecture number 1. The concept of resuscitation Resuscitation is a branch of clinical medicine that studies the problems of revitalizing the body, developing principles for the prevention of terminal conditions, methods of resuscitation and intensive care. Practical methods of revitalizing the body are united by the concept of "resuscitation". Resuscitation (from Latin "revival" or "animation") is a system of measures aimed at restoring sharply impaired or lost vital functions of the body and removing it from a terminal state and clinical death. Effective resuscitation measures are indirect heart massage and artificial ventilation of the lungs. If they are ineffective within 30 minutes, biological death is ascertained. Intensive care is a set of measures used to treat severe, life-threatening conditions and involves the use of a wide range of therapeutic measures, according to indications, including intravenous infusions, prolonged artificial ventilation of the lungs, pacing, dialysis methods, etc. A critical state is the impossibility of maintaining the integrity of body functions as a result of an acute dysfunction of an organ or system, requiring drug or hardware-instrumental replacement. The terminal state is a borderline state between life and death, a reversible extinction of body functions, including the stages of preagony, agony and clinical death. Clinical death is a terminal condition in which there is no blood circulation and respiration, the activity of the cerebral cortex stops, but metabolic processes are preserved. With clinical death, the possibility of effective resuscitation remains. The duration of clinical death is from 5 to 6 minutes. Biological death is an irreversible cessation of physiological processes in organs and tissues, in which resuscitation is impossible. It is established by a combination of a number of signs: the absence of spontaneous movements, contractions of the heart and pulse in large arteries, respiration, reactions to painful stimuli, corneal reflex, maximum pupil dilation and the absence of their reaction to light. Reliable signs of the onset of death are a decrease in body temperature to 20 ° C, the appearance of cadaveric spots and muscle rigor mortis. Lecture number 2. Basic manipulations in intensive care Percutaneous puncture and catheterization of the main vein (subclavian). Indications: large volumes of infusion-transfusion therapy, parenteral nutrition, detoxification therapy, intravenous antibiotic therapy, probing and contrasting of the heart, measurement of CVP, implantation of a pacemaker, impossibility of catheterization of peripheral veins. Contraindications: violation of the blood coagulation system, inflammatory and purulent process at the site of puncture and catheterization, trauma in the clavicle, superior vena cava syndrome, Paget-Schretter syndrome. Instrumentation and accessories for puncture and catheterization: puncture needle, a set of plastic catheters, a set of conductors, a 10 ml syringe for intramuscular injections, scissors, a needle holder, a surgical needle and a silk ligature, an adhesive plaster. Technique. Catheterization is carried out in compliance with the rules of asepsis and antisepsis, the processing of the operator's hands, the operating field and the application of sterile material. The position of the patient is horizontal on the back with the arms brought to the body and the lapel of the head in the opposite direction. Local anesthesia is used - 0,5-1% novocaine solution. The puncture is best done on the right, since when puncturing the left subclavian vein, there is a risk of damaging the thoracic lymphatic duct. Puncture point - on the border of the inner and middle third of the clavicle 2 cm below it. The needle is passed slowly at an angle of 45° to the collarbone and 30-40° to the surface of the chest between the collarbone and the 15st rib in the direction of the upper edge of the sternoclavicular joint. When passing the needle, the syringe plunger is periodically tightened to determine if it enters the vein, and novocaine is injected along the needle. When piercing a vein, sometimes there is a feeling of failure. After entering the vein, the syringe is disconnected from the needle and the cannula is closed with a finger. Then a conductor is inserted through the needle to a length of 20-6 cm and the needle is removed. A catheter of the appropriate diameter is passed through the conductor and, together with the conductor, is inserted into the vein by 8-XNUMX cm, after which the conductor is carefully removed. To check the correct position of the catheter, a syringe is attached to it and 2-3 ml of blood is drawn into it, after which a plug is placed or infusion therapy is started. The catheter is fixed with a silk ligature to the skin. To do this, a sleeve of adhesive plaster is made on the catheter 3-5 mm from the skin, on which silk is tied, then passed through the ears of the catheter and tied again. After fixing the catheter, the puncture site is closed with an aseptic sticker. Complications: puncture of the subclavian artery, air embolism, puncture of the pleural cavity, damage to the brachial plexus, damage to the thoracic lymphatic duct, damage to the trachea, goiter and thyroid gland, suppuration at the puncture site. 1. Tracheostomy Indications: obstruction of the larynx and upper trachea due to obstruction by a tumor or foreign body, paralysis and spasm of the vocal cords, severe swelling of the larynx, acute respiratory distress, aspiration of vomit, prevention of asphyxia in severe chest injuries. Tools: 2 scalpels, 2 anatomical and surgical tweezers, several hemostatic clamps, an elevator, a grooved probe, 2 blunt and 1 single-toothed sharp hook, Trousseau or Deschamps dilator, surgical needles with a needle holder. Technique The patient lies on his back, a roller under his shoulders, his head is thrown back. If the patient is in a state of asphyxia, the roller is placed only at the last moment, before opening the trachea. Local infiltration anesthesia is performed with a 0,5-1% solution of novocaine with the addition of adrenaline. In acute asphyxia, it is possible to operate without anesthesia. Identification points: the angle of the thyroid cartilage and the tubercle of the arch of the cricoid cartilage. An incision of the skin, subcutaneous tissue and superficial fascia is made from the lower edge of the thyroid cartilage to the jugular notch strictly along the midline of the neck. The median vein of the neck is retracted or ligated, finding a white line, along which the muscles are pushed apart in a blunt way and the isthmus of the thyroid gland is exposed. The edges of the incision are moved apart with a Trousseau dilator, ligatures are applied to the edge of the wound and the tracheostomy tube is carefully inserted, making sure that its end enters the lumen of the trachea. The surgical wound is sutured. The tube is fixed on the patient's neck with a gauze splint, previously tied to the tube shield. Insert the inner tube into the outer tube. 2. Conicotomy The patient is placed on his back with a transverse roller at the level of the shoulder blades. The patient's head is tilted back. After treating the skin on the front surface of the neck with an antiseptic solution, the larynx is fixed with fingers on the lateral surfaces of the thyroid cartilage and the gap between the thyroid and cricoid cartilage, where the cone-shaped ligament is located, is felt. Under local infiltration anesthesia with a pointed scalpel, a transverse skin incision about 2 cm long is made, the cone-shaped ligament is felt for and dissected or perforated. Any tracheostomy cannula of suitable diameter is inserted into the hole formed and fixed with a gauze strip around the neck. In the absence of a cannula, it can be replaced by a piece of rubber or plastic tube of suitable diameter and length. To prevent this tube from slipping into the trachea, its outer end is pierced transversely at a distance of 2 cm from the edge and fixed with a gauze strip. Conicotome is a small diameter metal tracheostomy cannula with a piercing mandrel inside it. After dissection of the skin over the cone-shaped ligament, it is pierced with a conicotome, the mandrel is removed, and the cannula is placed in a position that ensures free flow of air into the trachea and fixed. In extreme cases, with obstruction of the entrance to the larynx and a sharp violation of the airway, it can be restored by injecting 1-2 thick needles with an internal diameter of 2-2,5 mm into the trachea along the midline below the level of the thyroid cartilage. The needles are inserted at an acute angle to the tracheal axis, sometimes without local anesthesia, to a depth of 1-1,5-2 cm. . 3. Puncture of the pleural cavity Indications: sharply shortness of breath due to compression of the lungs by a massive effusion with pleurisy or hydrothorax, as well as air with valvular pneumothorax. Technique The puncture is carried out in a sitting position, under aseptic conditions. For anesthesia of the puncture site, a 0,5% solution of novocaine is used. A thick needle connected to a rubber tube is used for puncture. The puncture is made along the upper edge of the rib, since the intercostal vessels are located along the lower edge. The penetration of the needle into the pleural cavity is felt as a "failure into the void." Aspiration of fluid along the needle confirms that the end of the needle is in the pleural cavity. Each time a filled syringe is separated from the rubber tube, the latter must be clamped with a hemostatic clamp to prevent atmospheric air from being sucked into the pleural cavity. At the end of aspiration, an aseptic bandage is applied to the puncture site. Complications: injury to the intercostal artery, vessels of the diaphragm of the lung, puncture of the stomach or intestines. Tracheal intubation. Indications: narrowing of the larynx, pathological breathing, acute respiratory failure, coma II and III degree, high risk of aspiration during surgical interventions on the organs of the chest and abdominal cavity, head and neck, in diseases of the pharynx, larynx and trachea (acute inflammation, cancer, tuberculosis and etc.). A laryngoscope is used for intubation. It consists of a handle and a blade. The most widely used curved blades, as they are more physiological. Straight blades are used with a long neck. Preparation for intubation includes checking equipment and positioning the patient correctly. The endotracheal tube should be checked. The cuff is tested by inflating it with a 10 ml syringe. Check the contact of the blade with the handle of the laryngoscope and the light bulb. It is necessary to ensure that the suction is ready in case of sudden sputum discharge, bleeding or vomiting. Successful intubation depends on the correct position of the patient. The patient's head should be at the level of the xiphoid process of the intubator. Moderate head elevation with simultaneous extension at the atlantooccipital joint creates an improved position for intubation. Preparation for intubation also includes mandatory pre-oxygenation. The laryngoscope is held in the non-dominant hand (for most people, this is the left), and the patient's mouth is opened wide with the other hand. The blade is inserted along the right side of the oropharynx, avoiding damage to the teeth. The tongue is shifted to the left, and the blade is raised up to the arch of the pharynx. The tip of a curved blade is inserted into the vallecula (a fossa located on the anterior surface of the epiglottis), while the tip of a straight blade should lift the epiglottis directly. The handle of the laryngoscope is pushed up and forward perpendicular to the mandible until the vocal cords come into view. Reliance on teeth should be avoided. The endotracheal tube is taken in the right hand and passed through the open glottis under visual control. The cuff should be positioned in the upper trachea, but below the larynx. The laryngoscope is removed from the mouth, again avoiding damage to the teeth. Immediately after intubation, auscultation is performed over the lungs on both sides (since it is possible to pass a tube into one bronchus) and in the epigastrium (to exclude esophageal intubation). If the tube is in the trachea, it is fixed in position with ribbons and the cuff is inflated. The cuff should be positioned above the level of the cricoid cartilage, as long standing in the larynx can lead to hoarseness in the postoperative period. Complications: intubation of the esophagus, bronchus, location of the cuff in the larynx, damage to the teeth, dislocation of the lower jaw, laryngospasm, reflex disorders (hypertension, tachycardia, increased intracranial pressure), respiratory tract injury, inflammation, etc. 4. Puncture and catheterization of the epidural space Indications: severe pain syndrome, surgical interventions, providing postoperative analgesia. The level of epidural block setting depends on which organ needs to be anesthetized. Table No. 1 shows examples of "target organs" for epidural puncture. Table 1 Levels of the spinal column and "target organs"
Instrumentation: needles for anesthesia, a special needle for puncturing the epidural space, a sample syringe, a catheter, a plug, filter balls, napkins, adhesive tape and sterile gloves. The position of the patient is sitting or lying on his side. In this case, the knees and chin should be brought as close to the chest as possible. Thus, maximum flexion of the spine is created, at which the angle between the spinous processes of adjacent vertebrae increases and the approach to the yellow ligament is facilitated. Under aseptic conditions and under local anesthesia with a 0,5% solution of novocaine, a puncture of the epidural space is performed. The needle is injected strictly perpendicularly, but with osteochondrosis, an angle of inclination is possible or during puncture in the mid-thoracic region. When the needle enters the thickness of the ligaments, the mandrin is removed from it and a syringe with liquid is attached. Further advancement of the needle is carried out slowly and smoothly with pressure on the syringe plunger. Due to the significant resistance of the ligaments, the liquid cannot leave the syringe. The syringe is disconnected and the catheter is inserted 5-7 cm, there should be no resistance. The needle is removed and the guidewire is fixed to the back with adhesive plaster, bringing it to the front surface of the chest. The plug with the filter is fixed to the conductor. An anesthetic is injected. After that, the level of skin anesthesia is determined. Complications: respiratory and hemodynamic disorders, intoxication, damage to the dura mater, neurological complications, periduritis. 5. Lumbar puncture Indications: presence of meningeal syndrome, high intracranial pressure, differential diagnosis between ischemic and hemorrhagic stroke, traumatic brain injury, tumors of the spinal cord. Contraindications: the presence of an inflammatory or purulent process at the puncture site, hemorrhagic diathesis, tumor of the posterior cranial fossa, dislocation of the trunk, terminal state of the patient, with blurred boundaries of the optic nerve. The puncture point is between the 3rd and 4th spinous processes of the lumbar vertebrae. The manipulation is carried out under aseptic conditions, under local anesthesia. The needle goes perpendicular towards the navel. The laying of the patient is the same as with epidural puncture. With the passage of three ligaments (external and internal interspinous, yellow ligaments), there is a feeling of falling through, the mandrin is removed from the needle, and cerebrospinal fluid appears. After taking the cerebrospinal fluid for examination, a mandrin is inserted and the needle is removed, an aseptic sticker is applied. Unlike epidural puncture, damage to the dura mater occurs. The cerebrospinal fluid is clear, colorless, pressure 100-200 mm of water. Art., protein content 0,33 g / l, HC - 1003-1008, pH = 7,35-7,40, sugar content is equal to half of blood sugar (normally 2-3 mmol / l), chlorides - 110-120 mmol/l, the number of cells up to 5 lymphocytes. Complications: epiduritis, dislocation of the brain into the foramen magnum, neurological disorders. Lecture No. 3. Acute disorders of consciousness Consciousness is the highest form of reflection of reality, which is a set of mental processes that allow a person to navigate in the world around him, time, his own personality, which ensures his behavior. Impairment of consciousness is the general name for disorders of the integral activity of the brain, expressed in a violation of the ability to adequately perceive, comprehend and respond to the environment, navigate it, remember current events, make speech contact, and perform arbitrary expedient behavioral acts. There are various options for the oppression of consciousness (stupor, stupor, coma of various depths), as well as acute confusion (delirious state or metabolic encephalopathy). The degree of impaired consciousness varies from mild confusion to coma, and there are no clear transitions between these states. In practice, the degree of impaired consciousness is determined by the patient's reaction to stimuli. Stupefaction is a form of impaired consciousness, characterized by lethargy, slowing down and difficulty in the course of mental processes, rapid exhaustion of attention, an increase in the threshold for the perception of external stimuli, but while maintaining limited verbal contact. Stupefaction is based on a violation of attention, i.e., the ability to select the necessary information and coordinate responses in such a way that the logical sequence of thoughts and actions is not violated. The most common causes of stupor are metabolic and toxic disorders, but sometimes it is also observed with focal lesions of the cortex, especially the right parietal lobe. In such patients, it is possible to achieve a monosyllabic answer or the implementation of the simplest instructions only after persistent appeals to it or additional stimulation. With further oppression of consciousness, the possibility of speech contact is lost and sopor develops. Sopor is a state of deep depression of consciousness with the loss of the possibility of contact with the patient, but the preservation of coordinated defensive reactions and the opening of the patient's eyes in response to pain, sound or other stimuli. The patient cannot be fully awakened even with the help of painful stimuli, he lies with his eyes closed. The reaction to verbal instructions is weak or completely absent, it is impossible to get a response word or sound from the patient. With further oppression of consciousness, a coma develops. Coma is an unconscious state characterized by insensitivity to external stimuli. This is a life-threatening state of depression of the functions of the central nervous system and disorders of the regulation of vital functions. Coma can be caused by many different metabolic disorders and structural damage. Pathophysiology of coma Most often, coma is due to: 1) intracranial processes with damage to brain tissue (hematoma, abscess, tumor, epilepsy); 2) infectious lesions of the central nervous system (meningitis, encephalitis); 3) toxic damage to the brain (poisoning by alcohol, mushrooms, drugs); 4) failure of cerebral blood flow (consequences of asystole, Morgagni-Adams-Stokes attacks); 5) metabolic causes (impaired water and electrolyte balance, carbohydrate metabolism, acid-base balance, renal and hepatic insufficiency); 6) disorder of temperature balance (heat stroke, hypothermia). com classification According to etiology, the following coma is distinguished. 1. Primary, or intracranial: traumatic, vascular, infectious, neoplasms of the brain, epileptic, metabolic and hypoxic. 2. Secondary, or extracranial: severe brain injury. According to the severity of coma are classified in the following way. 1. Moderate coma, when the patient has a reaction to painful stimuli. In response to them, flexion and extensor movements may appear. But protective motor reactions are uncoordinated. The pain of the patient does not open his eyes. Pupillary and corneal reflexes are usually preserved, abdominal reflexes are depressed, and tendon reflexes are variable. Increased reflexes of oral automatism and pathological foot reflexes. 2. Deep coma. It is characterized by the absence of any reactions to any external stimuli, various changes in muscle tone, a decrease or absence of reflexes without bilateral mydriasis, disorders of spontaneous respiration and cardiovascular activity. 3. Terminal coma is determined by bilateral fixed mydriasis, diffuse muscle atony, severe violations of vital functions, rhythm and respiratory rate disorders, apnea, severe tachycardia; blood pressure is critical or not determined. Examination of a patient with a coma The plan of examination of the patient is as follows. 1. Assessment of the functional state of the respiratory and cardiovascular systems. 2. General clinical examinations, taking into account laboratory data, allowing to assess extracranial pathology. 3. Neurological examination. Laboratory studies: general clinical blood test (signs of a bacterial or viral infection); blood chemistry: glucose, coagulation factors (clotting time, prothrombin, fibrinogen, APTT, antithrombin III, paracoagulation tests, platelet count), urea, creatinine, bilirubin, ALT, AST, osmolarity, electrolytes (K, Na, Mg, Ca ); toxicological screening of blood, urine, gastric contents. Instrumental studies: radiography of the skull and cervical spine. Consultation of a neuropathologist (neurosurgeon) determines the further direction of the diagnostic search: computed or magnetic resonance imaging; EEG; ultrasound dopplerography. Lumbar puncture with analysis of cerebrospinal fluid is mandatory after: 1) consultation of an ophthalmologist and exclusion of signs of increased intracranial pressure - edema and elevation of the optic discs; 2) exclusion of signs of herniation of the brain. The following localizations of the herniation of the brain are distinguished. Diencephalic herniation, which occurs when the medial supratentorial localization is damaged and consists in the displacement of the diencephalon through the notch of the cerebellar tenon. This process calls: 1) Cheyne-Stokes breathing; 2) constriction of the pupils while maintaining their reaction to light; 3) paralysis of gaze up; 4) changes in mental status. The herniation of the medial parts of the temporal lobe, which occurs when the lateral supratentorial localization is affected, consists in the displacement of the medial parts of the temporal lobe through the notch of the cerebellar tenon. The resulting pressure on the structures of the midbrain is manifested by: 1) impaired consciousness; 2) an enlarged, non-reactive pupil on the side of the herniation, which is associated with compression of the III cranial nerve; 3) hemiparesis on the opposite side. The movements of the eyeballs are not always disturbed. Herniation of the tonsils of the cerebellum, which is caused by pressure pushing the lower part of the cerebellum through the foramen magnum, which leads to compression of the medulla oblongata. It causes: 1) impaired consciousness; 2) violations of the rhythm of breathing or apnea. Treatment Treatment should be as aggressive as possible and primarily aimed at ensuring adequate oxygenation and stabilization of central hemodynamics. If spontaneous breathing is maintained, humidified oxygen insufflation through a mask or nasal catheter is recommended. In the absence of spontaneous respiration or in the presence of pathological respiration, tracheal intubation is performed and the patient is transferred to artificial lung ventilation. With psychomotor agitation and reaction to mechanical ventilation, the use of sedatives (benzodiazepines, butyrophenones) is necessary. Stabilization of central hemodynamics is the normalization of blood pressure. In a hypertensive state, blood pressure must be reduced, but not more than 10% of the original per hour. A good effect is the use of sodium nitroprusside or magnesium sulfate. With hypotension, dopamine, dopamine, dobutrex and hormonal drugs are used. In the absence of anamnestic data and an unclear diagnosis, ex juvantibus therapy is performed (a positive response to drug exposure, on the one hand, gives the key to the diagnosis, on the other hand, it helps to gain time to avoid irreversible changes): 1) thiamine - 100 mg intravenously, subsequently - 100 mg intramuscularly (especially if there is a history of alcoholism, when determining high concentrations of ethanol in the blood); 2) glucose - a 40% solution of 60 ml intravenously (with an unknown level of glucose in plasma or at a level less than 3 mmol / l); 3) naloxone - 0,4-1,2 mg intravenously, fractionally, repeatedly, especially in the presence of "opiate signs" (traces of intravenous injections, narrow pupils, central respiratory disorders); 4) anexat (flumazenil) - 0,2 mg for 30 seconds, over the next minute, inject another 0,3 mg, over each next minute - 0,5 mg to a total dose of 3 mg. In the absence of an effect, it can be assumed that the coma is unlikely to be caused by benzodiazepine drugs; 5) in case of poisoning or overdose with a known drug or substance, it is necessary to administer the appropriate antidote (if there is a possibility of antidote therapy). Seizure control. Incoming brain hypoxia can cause status epilepticus. Seizure episodes may also result from anticholinesterase drug toxicity. For treatment, the drug of choice is benzodiazepines: midazolam (Dormikum) 5 mg intravenously in divided doses up to a total dose of 30 mg g, seduxen (Relanium) in divided doses up to 10 mg, intravenously. When status epilepticus develops, following benzodiazepines, it is necessary to administer phenytoin in a total dose of 1-1,5 g at a rate of 50 mg/min. If there is resistance to these drugs, it is necessary to administer phenobarbital (thiopental) in a total dose of up to 1000 mg by slow intravenous infusion (respiration and blood pressure control is necessary). For recurrent seizures, general anesthesia is necessary. In patients with EEG or computed tomography signs of an epileptic focus (hemorrhage, neoplasia, large ischemic infarction, abscess, etc.) and episodic epileptic seizures, maintenance therapy with phenytoin is required - 300 mg once a day per os. Maintaining normothermia. Control of rectal temperature is necessary: its decrease below 34 °C develops with hypothermia, overdose of sleeping pills and sedatives, hypothyroidism, Wernicke's disease. In these cases, it is necessary to gradually warm the patient to a temperature of 36 °C. Patients with hypothermia and lack of vital functions are subject to CPR, since low temperature reduces the demand for oxygen in the heart and brain and contributes to a better outcome of resuscitation measures (except for cases accompanied by hyperkalemia). The presence of fever in comatose patients requires an active search and treatment of infectious complications. The presence of signs of meningism may indicate the presence of either bacterial meningitis or subarachnoid hemorrhage (although approximately 12 hours must elapse between the onset of bleeding and chemical meningeal irritation). Another cause of fever may be an intracranial abscess or subdural hematoma. If bacterial meningitis is suspected, a lumbar puncture (cerebrospinal fluid analysis) and computed tomography should be performed to determine signs of increased intracranial pressure. Preventing aspiration of gastric contents. The need for gastric lavage in case of poisoning and drug overdose and, therefore, the installation of a gastric tube increases the risk of regurgitation of gastric contents (due to relaxation of the gastroesophageal sphincter). Therefore, before inserting a gastric tube, it is necessary to perform tracheal intubation with a sealing cuff, which is the best means of protecting the airway. Urological treatment. To control diuresis, it is necessary to install a Foley catheter, ensuring aseptic conditions and conducting antimicrobial therapy to prevent urogenital sepsis. Reduced intracranial pressure. An increase in ICP is a clinical emergency that requires the implementation of appropriate measures aimed at reducing it, which avoids secondary damage to the brain due to compression of its tissues or a decrease in cerebral blood flow. Carrying out the above diagnostic measures makes it possible to establish the causes of increased ICP, and, accordingly, the key measures are aimed at its elimination (operative and conservative treatment). Hyperventilation to maintain pCO levels2 25-30 mmHg Art. (levels less than 25 mm Hg can cause a significant decrease in cerebral blood flow, leading to cerebral ischemia). Restriction of fluid intake. It is necessary to exclude solutions containing free water (5% glucose). Isotonic NaCl solution, necessary to maintain blood osmolarity, should be administered at half the dose. Introduction of osmotically active substances. Mannitol is administered at a dose of 1-2 g/kg for 10-20 minutes, and then at a maintenance dose of 0,05-0,3 g/kg every 6 hours. Additionally, furosemide is administered to more effectively reduce ICP. Strict control of the therapy is necessary to prevent complications: a decrease in intravascular volume, hypotension, hypernatremia, hypocalcemia, hypokalemia, as well as a response syndrome and rupture of cortical veins in subdural hematoma. An important measure to prevent complications is to maintain systolic blood pressure at 100-110 mm Hg. Art. Drugs also lead to a decrease in ICP. The use of muscle relaxants helps to reduce ICP during mechanical ventilation (blockade of increased intrathoracic venous pressure during mechanical ventilation), but they are recommended only for a very short time. The use of corticosteroids is effective in cases of increased intracranial pressure due to neoplasia or focal ischemia (stroke) of the brain. The effectiveness of corticosteroids in the treatment of increased intracranial pressure due to trauma and general cerebral ischemia has not been proven. It is important to remember that glucocorticoids can cause an increase in blood glucose levels and, accordingly, increase cerebral ischemia. Types of com Hypoglycemic coma occurs with an overdose of insulin in the treatment of diabetes mellitus or with restriction of carbohydrate intake. The development of coma is preceded by bulimia, irritability, fear. Diplopia, hallucinations, tonic and clonic convulsions are sometimes noted. Excitation is replaced by adynamia and vice versa. The patient quickly loses consciousness and is covered with sweat. The skin is moist and pale, breathing is shallow, rhythmic. Sometimes spontaneous hypoglycemia is observed in athletes and after heavy physical exertion. If the hypoglycemic coma lasts more than 3 hours, the development of gross organic lesions of the central nervous system is possible. It is important to lower the blood sugar level below 3 mmol. There is no sugar or acetone in the urine. Treatment. Immediately enter 20-40% glucose at a dose of 20-30 ml intravenously as a bolus. After that, blood and urine sugar control is carried out. Diabetic coma, or hyperglycemic, when the blood glucose level is sharply increased. Coma is preceded by drowsiness, thirst, anorexia, nausea, vomiting, headache. Hyperglycemia, metabolic acidosis are determined in the laboratory, sugar and acetone are present in the urine (not always). The face is pale and hyperemic, the mucous membranes are dry, the skin is also dry, and its turgor is reduced. The eyeballs are sunken, the smell of acetone from the mouth is possible. Breathing is rare pathological. Hemodynamics is disturbed: tachycardia, arterial hypotension, muffled heart sounds. Treatment. Elimination of hypovolemia with the help of intravenous administration of sodium chloride in a volume of 3-5 liters per day. Insulin therapy consists in the introduction of short-acting insulin 6-10 IU per hour with an infusion pump. With a decrease in blood glucose to 11-13 mmol / l, the dose of insulin is reduced to 4-8 units per hour, and an infusion of 5% glucose begins to avoid a hypoglycemic state. Thyrotoxic coma is rare, but it should be considered if, with severe tachycardia, there are no typical signs of hemodynamic myocardial insufficiency and there is energy-dynamic heart failure. The presence of struma, eye glare, and tremor usually also draws attention to this possibility. The clinical picture should be supplemented by collecting anamnestic data, since studies confirming the diagnosis (basal metabolism, radioactive iodine) cannot be carried out. Alcohol intoxication is manifested by the smell of alcohol from the mouth, a delirious state, anxiety, vomiting, and a puffy face. Breathing is slow, pulse is quickened, pupils are dilated. In patients with alcoholism, delirium develops 2-3 days after alcohol withdrawal. The development of delirium is prevented by the use of benzodiazepines when warning signs (fever, tremor, tachycardia, hypertension) appear. With the development of delirium, the drugs of choice are: in young people, diazepam (intravenous administration), and in elderly patients and patients with impaired liver function, lorazepam, but if necessary, a quick effect is preferable to diazepam (5 mg every 5 minutes until the effect is achieved). Cases of the need to administer 2640 mg of diazepam for the treatment of a severe delirious state are described. Additionally, blockers and clonidine are used. Also in these conditions, the use of antipsychotics (haloperidol, droperidol) is useful. With apoplexy coma (develops with various intracerebral processes), the leading symptom is hemiplegia or paralysis of individual muscle groups. Paralysis appears when the eyes and head are turned in the opposite direction to the paralyzed: "the patient looks at the lesion in the brain." The mouth is skewed to the healthy side: "smoking a pipe on the diseased side." On the hemiplegic side, the elevated limb falls quickly and heavily onto the bed, while the unaffected limb slowly returns to its original position. Coma with Addison's disease (adrenal coma, often developing with adrenal tuberculosis, trauma, infectious diseases) is rare. The leading symptom is pathologically low, often unmeasurable blood pressure. Along with collapse, this symptom is caused by changes in carbohydrate metabolism (hypoglycemia), electrolyte imbalance and water metabolism. Suddenly there is a sharp pallor, cold sweat. Excitation is quickly replaced by adynamia, then the patient loses consciousness. Acrocyanosis appears, the skin becomes marbled. On the skin of the back and extremities, pigmentation is found in the form of dark spots and a bright red petechial rash. Heart sounds are muffled. Dehydration and oliguria quickly set in. In the blood, metabolic acidosis, hypoglycemia and an increase in residual nitrogen. Treatment consists in the rapid introduction of glucocorticosteroids at a dose of 1 mg / kg. The dose can be increased by 2-3 times. A similar dose is administered intramuscularly. To combat dehydration, an isotonic solution of sodium chloride is administered, and then glucose. Lecture number 4. Cardiopulmonary resuscitation Cardiopulmonary resuscitation (CPR) is a complex of surgical and therapeutic measures performed in the absence of life-threatening injuries and aimed at restoring and supporting the function of the cardiorespiratory system. Indications for cardiopulmonary resuscitation: carried out in patients with no effective pulse on the carotid arteries or a thready, weak pulse, who are unconscious and (or) in the absence of effective respiratory movements. The most common cases of primary cardiac arrest, as well as primary respiratory failure. Contraindications: trauma incompatible with life, terminal stages of incurable diseases and biological death. Basic principles Primary efforts in CPR are aimed at: 1) chest compression; 2) blowing air into the lungs and ventilation; 3) preparation and administration of drugs; 4) installation and maintenance of intravenous access; 5) specialized activities (defibrillation, pacemaker installation, tracheal intubation). Thus, to complete the full scope of activities, 4 people and a team leader are needed. One person should be in charge of CPR. This person should integrate all available information and prioritize impact. He must monitor the ECG monitor, the use of drugs and ensure that the actions of other team members are corrected. He should be removed from the performance of procedures that detract from the leadership role. For more than 40 years, the Safar resuscitation alphabet has been used for CPR. In this complex, the sequence of actions of the resuscitator is sustained; according to their English name, they are indicated by the corresponding letters. A - Airway - ensuring airway patency. B - Breathing - artificial ventilation of the lungs (ALV) in an accessible way, for example, when breathing "mouth to mouth". C - Circulation - ensuring hemocirculation - indirect heart massage. D - Drugs - the introduction of drugs. E - Electrocardiography - ECG registration. F - Fibrilation - conducting, if necessary, electrical defibrillation (cardioversion). G - Gauging - evaluation of primary results. H - Hypothermy - head cooling. I - Intensive care - intensive care for post-resuscitation syndromes. A - Airway - airway management The patient is placed horizontally on his back. The head is thrown back as much as possible, for this the doctor puts one hand under the neck, the other is placed on the patient's forehead; a test breath is taken from mouth to mouth. If a patient with reduced muscle tone lies on his back, his tongue may sink, as if packing the throat. At the same time, the epiglottis descends, further blocking the airways. Appear: sonorous breathing, then violations of the respiratory rhythm up to its complete stop. Such phenomena develop especially rapidly in patients who are unconscious. To prevent and eliminate the retraction of the tongue, the lower jaw should be brought forward and at the same time hyperextension in the occipito-cervical joint should be performed. To do this, with the pressure of the thumbs on the chin, the lower jaw of the patient is shifted down, and then with the fingers placed at the corners of the jaw, they push it forward, supplementing this technique with overextension of the head posteriorly (triple Safar technique). With the correct and timely conduct of these manipulations, the patency of the airways at the level of the pharynx is quickly restored. Foreign bodies (blood clots, mucus, dentures, etc.) can be the cause of airway obstruction. They are quickly removed with any improvised materials (napkin, handkerchief). The patient's head should be turned to the side due to the danger of aspiration. The restoration of patency of the upper respiratory tract is facilitated by the use of various air ducts. The most appropriate is the use of an S-shaped duct. For its introduction, the patient's mouth is opened with crossed fingers II and I, and the tube is advanced to the root of the tongue so that its opening "slides" along the palate. Care must be taken to ensure that the air duct does not move during transport. If all the described procedures are not effective, then we can assume the presence of obturation of the airways in the underlying sections. In these cases, direct laryngoscopy and active aspiration of pathological secretion is required, followed by tracheal intubation for 10-15 seconds. It is advisable to perform conicotomy and tracheostomy. B - Breathing - artificial lung ventilation (ALV) in an accessible way The simplest and most effective method of artificial respiration during resuscitation is the "mouth-to-mouth" method, when the resuscitator's exhaled air is blown into the victim's lungs under pressure. Having thrown back the head of the victim, with one hand they pinch his nostrils, put the other hand under his neck, take a deep breath, tightly pressing his lips to the lips of the victim (in children, to the lips and to the nose at the same time) and blow air into the lungs of the victim, observing the rise of the chest during inhalation time. As soon as the chest rises, the air injection is stopped, they move their face to the side, they take a deep breath again, and the patient at this time has a passive exhalation. After 2-3 inflations of the lungs, the presence of a pulse on the carotid artery is determined, if it is not detected, then they proceed to artificial restoration of blood circulation. Manual ventilation is used using a self-expanding Ambu-type bag. When using a ventilator, the respiratory rate is 12-15 per minute, the inspiratory volume is 0,5-1,0 liters. In a hospital, tracheal intubation is performed and the patient is transferred to a ventilator. C-Circulation - ensuring hemocirculation - indirect heart massage Closed heart massage is the simplest and most efficient way of emergency artificial circulatory support. Closed heart massage should be started immediately, as soon as the diagnosis of acute circulatory arrest is made, without clarifying its causes and mechanisms. In cases of ineffective heart contractions, one should not wait for a complete cardiac arrest or an independent restoration of adequate cardiac activity. Basic rules for closed heart massage. 1. The patient should be in a horizontal position on a solid base (floor or low couch) to prevent the possibility of displacement of his body under the strengthening of the massaging hands. 2. The zone of application of the force of the hands of the resuscitator is located on the lower third of the sternum, strictly along the midline; the resuscitator can be on either side of the patient. 3. For massage, one palm is placed on top of the other and pressure is applied to the sternum in the area located 3-4 transverse fingers above the place of attachment to the sternum of the xiphoid process; the hands of the massager, straightened at the elbow joints, are positioned so that only the wrist produces pressure. 4. Compression of the victim's chest is performed due to the gravity of the doctor's torso. The displacement of the sternum towards the spine (i.e., the depth of the deflection of the chest) should be 4-6 cm. 5. The duration of one chest compression is 0,5 s, the interval between individual compressions is 0,5-1 s. Rate of massage - 60 massage movements per minute. In intervals, the hands are not removed from the sternum, the fingers remain raised, the arms are fully extended at the elbow joints. When resuscitation is carried out by one person, after two quick injections of air into the lungs of the patient, 15 chest compressions are performed, i.e. the ratio "ventilation: massage" is 2: 15. If 2 persons are involved in resuscitation, then this ratio is 1: 5, i.e., there are 5 chest compressions per breath. A prerequisite for cardiac massage is the constant monitoring of its effectiveness. The criteria for the effectiveness of massage should be considered as follows. 1. Change in skin color: it becomes less pale, gray, cyanotic. 2. Constriction of the pupils, if they were dilated, with the appearance of a reaction to light. 3. The appearance of a pulse impulse on the carotid and femoral arteries, and sometimes on the radial artery. 4. Determination of blood pressure at the level of 60-70 mm Hg. Art. when measured at the shoulder. 5. Sometimes the appearance of independent respiratory movements. If there are signs of restoration of blood circulation, but in the absence of a tendency to preserve independent cardiac activity, heart massage is performed either until the desired effect is achieved (restoration of effective blood flow), or until the signs of life disappear permanently with the development of symptoms of brain death. In the absence of signs of restoration of even reduced blood flow, despite heart massage for 25-30 minutes, the patient should be recognized as dying and resuscitation measures can be stopped. D - Drugs - drug administration In case of acute cessation of blood circulation, the introduction of agents that stimulate cardiac activity should begin as soon as possible, if necessary, be repeated during resuscitation. After the start of cardiac massage, 0,5-1 ml of adrenaline should be administered as soon as possible (intravenously or intratracheally). Its repeated introductions are possible after 2-5 minutes (up to 5-6 ml in total). With asystole, adrenaline tones the myocardium and helps "start" the heart, with ventricular fibrillation it contributes to the transition of small-wave fibrillation to large-wave, which greatly facilitates defibrillation. Adrenaline facilitates coronary blood flow and increases the contractility of the heart muscle. Instead of epinephrine, isodrin can be used, which is 3 times more effective than adrenaline in terms of the effectiveness of the effect on the myocardium. The initial dose is 1-2 ml intravenously, and the next 1-2 ml in 250 ml of a 5% glucose solution. In conditions of impaired blood circulation, metabolic acidosis progressively increases, therefore, immediately after the infusion of adrenaline, a 4-5% solution of sodium bicarbonate is administered intravenously at the rate of 3 ml / kg of the patient's body weight. In the process of dying, the tone of the parasympathetic nervous system increases significantly, the brain is depleted, therefore, M-cholinolytics are used. With asystole and bradycardia, atropine is administered intravenously in a 0,1% solution - 0,5-1 ml, up to a maximum dose of 3-4 ml. To increase myocardial tone and reduce the effect of hyperkalemia, intravenous administration of 5 ml of a 10% solution of calcium chloride is recommended. Adrenaline, atropine and calcium chloride can be administered together in the same syringe. With severe tachycardia and especially with the development of fibrillation, the use of lidocaine at a dose of 60-80 mg is indicated, but since it is short-acting, it is infused at a rate of 2 mg / min. It is also indicated to use glucocorticoids, which, by increasing the sensitivity of adrenoreactive myocardial structures to catecholamines and normalizing the permeability of cell membranes, contribute to the restoration of adequate cardiac activity. E - Electrocardiography - ECG recording With the help of an ECG study, the nature of the violation of cardiac activity is determined. Most often it can be asystole - complete cessation of heart contractions, fibrillation - chaotic uncoordinated contraction of myocardial fibers with a frequency of 400-500 beats / min, in which cardiac output practically stops. Initially, large-wave fibrillation is noted, which, within 1-2 minutes, passes into small-wave fibrillation, followed by asystole. The presence of any rhythm on the ECG is better than the complete absence of electrical activity of the myocardium. Therefore, the key task of CPR is to stimulate the electrical activity of the myocardium and subsequently modify it into an effective (presence of a pulse) rhythm. The presence of asystole serves as a marker of severe myocardial perfusion disorder and serves as a poor prognostic sign for restoring cardiac rhythm. However, it is important to differentiate between low-amplitude microwave ventricular fibrillation and asystole, which is best done in standard ECG leads 2-3. Adrenaline (1 mg intravenously) and atropine (1 mg increased to 2-4 mg) are most effective in restoring electrical activity. In refractory cases, correction of potassium and calcium levels is effective. Ventricular fibrillation (VF) In pulseless patients, immediate blind electropulse therapy should be performed (before the cause of circulatory arrest is recognized by ECG), since VF is the most common cause of sudden death, and the success of defibrillation is largely determined by the time it is performed. It should be noted that "blind" defibrillation will not harm patients with asystole and bradycardia and is usually effective in patients with tachycardia and VF. It is important to remember that the rule of "blind" cardioversion is not acceptable in children, since they are much more likely than VF to have respiratory arrest as a cause of terminal illness. The success of defibrillation depends on VF amplitude, which in turn is inversely correlated with the duration of the VF episode. If two initial attempts at cardioversion are ineffective, in this case it is necessary to administer adrenaline to increase the amplitude of fibrillation waves and increase vascular tone (in cases of restoration of the heart rhythm, it allows increasing perfusion of the heart and brain). On the other hand, it is necessary to use optimal doses of adrenaline so as not to increase the oxygen demand of the myocardium. F - Fibrilation - performing electrical defibrillation if necessary (cardioversion) Cardiac fibrillation can be eliminated by the use of electrical defibrillation. It is necessary to apply electrodes tightly to the chest (in the anterolateral position, one electrode is located in the region of the apex of the heart, the second in the subclavian region to the right of the sternum), which increases the force of the discharge and, accordingly, the effectiveness of defibrillation. In a number of patients, the anteroposterior (apex of the heart - interscapular space) position of the electrodes is more effective. Do not apply electrodes over the overlays of the ECG monitor. It should be noted that electrical defibrillation is effective only when large-wave oscillations with an amplitude of 0,5 to 1 mV or more are recorded on the ECG. This kind of myocardial fibrillation indicates the safety of its energy resources and the possibility of restoring adequate cardiac activity. If the oscillations are low, arrhythmic and polymorphic, which is observed in severe myocardial hypoxia, then the possibility of restoring cardiac activity after defibrillation is minimal. In this case, with the help of heart massage, mechanical ventilation, intravenous administration of adrenaline, atropine, calcium chloride, it is necessary to achieve the transfer of fibrillation to large-wave, and only after that defibrillation should be performed. The first attempt at defibrillation is carried out with a discharge of 200 J, with subsequent attempts the charge increases to 360 J. The electrodes must be moistened and firmly pressed to the surface of the chest. The most common errors during defibrillation, which cause the ineffectiveness of the latter, include the following. 1. Long interruptions in heart massage or complete absence of resuscitation during the preparation of the defibrillator for discharge. 2. Loose pressing or insufficient moistening of the electrodes. 3. Application of a discharge against the background of low-wave fibrillation without taking measures that increase the energy resources of the myocardium. 4. Applying a discharge of low or excessively high voltage. It should be noted that electrical defibrillation of the heart is an effective method for correcting such cardiac arrhythmias as paroxysmal ventricular tachycardia, atrial flutter, nodal and supraventricular tachycardia, atrial fibrillation. The indication for electrical defibrillation, at the prehospital stage, is most often paroxysmal ventricular tachycardia. A feature of defibrillation in these conditions is the presence of consciousness in the patient and the need to eliminate the reaction to pain when applying an electric discharge. G - Gauging - evaluation of primary results The primary evaluation of the results is carried out not only to ascertain the state of the circulatory and respiratory system, but also in order to outline the tactics of further therapeutic measures. Upon completion of the resuscitation process, in which the restoration of cardiac activity appeared, the resuscitator must perform a number of final actions: 1) assess the condition of the respiratory tract (symmetry of breathing, with the continuation of forced breathing, the adequacy of ventilation); 2) check the pulsation in the central and peripheral arteries; 3) evaluate the color of the skin; 4) determine the level of blood pressure; 5) measure the volume of circulating blood (measure CVP, assess the condition of the jugular veins); 6) check the correct position of the catheters in the central veins; 7) in case of elimination of cardiac fibrillation, which was the cause of sudden death, make sure that the infusion of any antifibrillary agent is continued; 8) carry out correction of therapy if it was carried out to the patient before the episode of sudden death. H - Hypothermy - head cooling With hypothermia, the critical time of circulatory arrest can increase significantly. To prevent the development of posthypoxic encephalopathy, measures should be taken to reduce the intensity of metabolic processes in the brain, as well as antihypoxic and antioxidant drugs. Key events 1. Craniocerebral hypothermia - wrapping the head and neck with ice packs, snow, cold water. 2. Parenteral administration of antihypoxants (sodium oxybutyrate, mafusol, small doses of sedatives), as well as improving the rheological properties of blood (rheopolyglucin, hemodez, heparin, trental). 3. The introduction of calcium antagonists (nimoton, lidoflazin, etc.). 4. Introduction of antioxidants (mafusol, unitiol, vitamin C, catalase, etc.). I - intensive care - conducting intensive care of postresuscitation syndromes Although a rapid positive response to CPR improves the chances of a favorable prognosis in patients, subsequent development of sepsis, acute pulmonary insufficiency and pneumonia is possible, which naturally worsens the prognosis. Long-term survival of patients with previous diseases of vital organs after CPR is not typical, since during this period their lesions deepen, and the nerve centers that provide autonomous control and maintenance of protective reflexes are damaged. Also, when intensive chest compression is used, ruptures of the liver, aorta, pneumothorax, fractures of the ribs and sternum are noted. Frequent complications are aspiration pneumonitis, convulsions (due to cerebral ischemia) and lidocaine intoxication. A number of patients develop bleeding from stress ulcers of the stomach and duodenum. After CPR, there is a significant increase in the level of liver (and/or skeletal muscle) enzymes, although the development of liver necrosis and insufficiency of its function are rare. In high-energy defibrillation regimens, there is a significant increase in the level of creatine phosphokinase, but an increase in the MB fraction is present only with repeated high-energy discharges. 1. Correction of CBS and water-electrolyte balance. Often after CPR, metabolic alkalosis, hypokalemia, hypochloremia, and other electrolyte disorders develop. There is a shift in pH to an acidic or alkaline environment. The key to pH correction is adequate ventilation. The use of bicarbonate should be carried out under the control of the gas composition of the blood. As a rule, there is no need to introduce NSO3 with the rapid restoration of blood circulation and respiration. With a functioning heart, a pH level of ~ 7,15 is adequate for the functioning of the cardiovascular system. The commonly recommended dose of bicarbonate (1 mg/kg) may cause side effects including: 1) arrhythmogenic alkalosis; 2) increased CO production2; 3) hyperosmolarity; 4) hypokalemia; 5) paradoxical intracellular acidosis of the central nervous system; 6) shift to the left of the hemoglobin dissociation curve, limiting the tissue supply of O2. Therefore, the appointment of this drug should be strictly according to indications. To eliminate hypokalemia, an intravenous infusion of potassium chloride is performed at a dose of 2 mmol/kg per day. 2. Normalization of the antioxidant defense system. Intensive therapy includes a complex of antioxidant drugs with multidirectional action - mafusol, unitiol, vitamin C, multibiont, tocopherol, probucol, etc. 3. The use of antioxidants helps to reduce the intensity of metabolic processes and, consequently, reduce the need for oxygen and energy, as well as the maximum use of the reduced amount of oxygen that is available during hypoxia. This is achieved through the use of neurovegetative protection drugs and antihypoxants (seduxen, droperidol, ganglion blockers, mexamine, sodium hydroxybutyrate, cytochrome, gutimin, etc.). 4. An increase in energy resources is provided by intravenous administration of concentrated glucose solutions with insulin and the main coenzymes involved in energy utilization (vitamin B6, cocarboxylase, ATP, riboxin, etc.). 5. Stimulation of the synthesis of protein and nucleic acids - substrates that are absolutely necessary for the normal functioning of cells, the synthesis of enzymes, immunoglobulins and others, is carried out by the use of anabolic hormones (retabolil, nerabolil, insulin, retinol), folic acid, as well as the introduction of amino acid solutions. 6. Activation of aerobic metabolism is achieved by introducing a sufficient amount of oxidation substrates (glucose), as well as using hyperbolic oxygenation (HBO) - this method ensures the supply of the required amount of oxygen even in conditions of sharp violations of its delivery. 7. Improvement of redox processes (succinic acid, riboxin, tocopherol, etc.). 8. Active detoxification therapy contributes to the normalization of metabolic processes. For this, various methods of infusion therapy (gelatinol, albumin, plasma), forced diuresis, etc. are used. In severe cases, extracorporeal detoxification methods are used (hemosorption, hemodialysis, plasmapheresis). 9. Elimination of violations of microcirculation processes. For this, heparin therapy is performed. There is no single guideline for all clinical situations. During ongoing CPR, neurological signs cannot serve as markers of outcome and, accordingly, cannot be guided by them when CPR is stopped. Resuscitation is rarely effective if more than 20 minutes is needed to restore a coordinated heart rhythm. A number of studies have shown that the lack of response within 30 minutes to full CPR, with rare exceptions, leads to death. The best results occur in cases of immediate effective cardioversion. Prolonged resuscitation with a good neurological outcome is possible with hypothermia and deep pharmacological depression of the central nervous system (for example, barbiturates). Methods for determining the non-viability of the brain: 1) angiography of cerebral vessels (lack of blood flow); 2) EEG (straight line for at least 24 hours); 3) computed tomography. CPR Termination Criteria: 1) if within 30 minutes all correctly performed resuscitation measures do not bring any effect - spontaneous breathing does not appear, blood circulation is not restored, the pupils remain dilated and do not react to light; 2) if within 30 minutes there are repeated cardiac arrests that are not amenable to therapy, and at the same time there are no other signs of successful resuscitation; 3) if in the process of resuscitation it was found that this patient was not shown at all; 4) if within 45-60 minutes, despite the partial restoration of breathing, the victim has no pulse and there are no signs of restoration of brain function. Lecture No. 5. Emergency conditions in pulmonology Acute respiratory failure is a pathological condition of the body in which the function of the external respiration apparatus is insufficient to provide the body with oxygen and adequate removal of carbon dioxide. Normal tidal volume (TO) is 500 ml (alveolar ventilation - 350 ml, dead space 150 ml). Minute volume of ventilation (MOV) - 6-8 l. Oxygen consumption - 300 ml/min. In the exhaled air, oxygen is 16%, in the inhaled - 21%. Oxygen in the inhaled mixture should be at least 20%. Causes of acute respiratory failure: a violation of the central regulation of breathing or a mismatch between ventilation and blood flow at the level of respirons - the final structural and functional units of the lungs. Overdose of narcotic substances (inhalation), narcotic analgesics, acute cerebral edema, cerebrovascular accident, brain tumors, reduced airway lumen or complete obstruction, retraction of the tongue, a large amount of sputum, especially in patients with suppurative lung diseases (abscess, bilateral bronchiectasis), pulmonary hemorrhage, vomiting and aspiration, laryngospasm and bronchospasm. When the tongue is retracted, an air duct should be placed or it is most reliable to intubate and artificially ventilate. With the accumulation of sputum, it is necessary to force the patient to expectorate it. If the patient is unconscious, then the respiratory tract is sanitized. In severe patients, anesthesia and active sanitation are performed. Catheterization of the trachea, bronchial tree and removal of the contents are performed. 1. Laryngospasm Laryngospasm is the closure of the true and false vocal cords. In both cases, control agents (eufillin) are necessarily used. If this does not help, it is necessary to introduce short-acting muscle relaxants, intubate and transfer the patient to mechanical ventilation. Muscle relaxants cause respiratory failure in the postoperative period if sufficient decurarization is not performed. It is usually produced by anticholinesterase drugs (prozerin). By the time of extubation, it is necessary to make sure that strength and muscle tone have recovered (ask to raise a hand, squeeze a hand, raise a head). With multiple fractures of the ribs, part of the chest sinks during inhalation, the so-called paradoxical breathing develops, so it is necessary to restore the chest frame. For this patient, it is necessary to intubate, after introducing relaxants, with further transfer to mechanical ventilation (until the integrity of the chest is restored). The following leads to a decrease in the functioning lung parenchyma: atelectasis, lung collapse, pneumonia, the consequences of surgery, pneumo-, hemo-, pyothorax. Differences between atelectasis and collapse: atelectasis is an obstruction in a straightened state. This condition is characterized by the presence of an unventilated lung through which half of the circulating blood passes, the latter is not oxygenated. As a result, acute respiratory failure develops. When the lung collapses, it is compressed by air or fluid in the pleural cavity. At the same time, blood circulation in the compressed lung decreases sharply, and blood circulation in a healthy lung increases. Therefore, collapse is not as dangerous a complication in terms of the development of acute respiratory failure as atelectasis. Before surgery, the function of the intact lung should be assessed (separate spirography). According to the stage of development, acute respiratory failure is divided into: 1) dysfunction; 2) insufficiency; 3) failure of prosthetic function. According to the rate of development, acute respiratory failure is divided into: 1) lightning fast (develops within a minute); 2) acute (develops within a few hours); 3) subacute (develops within a few days); 4) chronic (lasts for years). The main elements of intensive care for acute respiratory failure: oxygen therapy, drainage position of the patient, fibrobronchoscopy, tracheostomy, intubation and mechanical ventilation, bronchodilation, hormone therapy, HBO. 2. Pulmonary embolism Pulmonary embolism (PE) is a blockage of the main or middle trunk, small vascular trunks of the pulmonary artery, leading to an increase in pressure in the pulmonary circulation, right ventricular failure. Predisposing factors Diseases of the cardiovascular system - atherosclerosis, rheumatic heart disease, rheumatic malformations, septic endocarditis. Diseases of the veins of the lower extremities, pathology of the organs and vessels of the small pelvis. Postoperative PE in particular require close attention. Most often, embolism develops during operations on: vessels of the lower extremities, bladder, female genital organs, prostate gland, pelvic bones and hip joint. Changes in the system of hemostasis, spontaneous fibrinolysis, retraction and organization of venous thrombi are essential. Patients with oncological diseases, obesity, circulatory insufficiency, who are forced to stay in bed for various reasons, are also at the greatest risk. Clinical classification of PE Form: heavy, medium and light. Downstream: fulminant, acute, recurrent. According to the level of damage to the pulmonary artery: trunk or main branches, lobar (segmental) branches, small branches. Clinic and diagnostics The clinical course of PE is quite variable. The most common symptoms are sudden onset of shortness of breath (RR ranges from 30 to more than 50 per minute), rapid breathing, pallor, more often cyanosis, swelling of the jugular veins, tachycardia, arterial hypotension (up to shock), retrosternal pain, cough and hemoptysis. Auscultation often determines the strengthening of the II tone over the pulmonary artery. X-ray signs - an increase in the size of the proximal pulmonary artery, depletion of the peripheral pattern, as well as raising the dome of the diaphragm. The ECG may reveal overload of the right departments (cor pulmonale): 1) the appearance of Q waves with a simultaneous increase in the amplitude of the R and S waves (QS syndrome); 2) rotation of the heart around the longitudinal axis with the right ventricle forward (shift of the transition zone to the left chest leads); 3) ST segment elevation with negative T wave in leads III, aVF, V1-V3; 4) the appearance or increase in the degree of blockade of the right leg of the bundle of His; 5) high pointed "pulmonary" tooth P with a deviation of its electrical axis to the right; 6) sinus tachycardia or tachysystolic form of atrial fibrillation. Echocardiography allows detecting acute cor pulmonale, determining the severity of hypertension in the pulmonary circulation, assessing the structural and functional state of the right ventricle, detecting thromboembolism in the heart cavities and in the main pulmonary arteries, visualizing an open foramen ovale, which can affect the severity of hemodynamic disorders and be the cause of paradoxical embolism . However, a negative echocardiographic result by no means rules out the diagnosis of pulmonary embolism. The most informative diagnostic method is pulmonary artery angiography. For preventive purposes, anticoagulants are used in the postoperative period. The dose of heparin is 10 IU per day (000 IU 2 times). In the presence of contraindications, anticoagulants are not prescribed. Contraindications include: severe brain damage; oncopathology with the potential for bleeding; thrombocytopenia; pulmonary tuberculosis; severe chronic diseases of the parenchyma of the liver and kidneys with functional insufficiency. Treatment Anticoagulant therapy. Anticoagulants can prevent secondary thrombosis in the pulmonary vascular bed and the progression of venous thrombosis. It is advisable to widely use low molecular weight heparins (dalteparin, eioxaparin, fraxiparin), which, in comparison with conventional unfractionated heparin, rarely cause hemorrhagic complications, have less effect on platelet function, have a longer duration of action and high bioavailability. thrombolytic therapy. In massive PE, thrombolytic therapy is indicated and justified in cases where the volume of the lesion is relatively small, but pulmonary hypertension is pronounced. Most often, streptokinase is used at a dose of 100 units per hour. But one should be aware of severe allergic reactions. The duration of thrombolysis is usually 000-1 days. Urokinase and alteplase are devoid of antigenic properties, but have high resistance. Surgery. Embolectomy is indicated for patients with thromboembolism of the pulmonary trunk or both of its main branches with an extremely severe degree of impaired lung perfusion, accompanied by pronounced hemodynamic disorders. All manipulations to remove emboli after cross-clamping of the vena cava should last no more than 3 minutes, since this interval is critical for patients who are operated on under conditions of severe initial hypoxia. It is optimal to perform embolectomy under cardiopulmonary bypass using transsternal access. 3. Bronchial asthma Bronchial asthma is a disease based on chronic inflammation of the airways with an autoimmune component, accompanied by a change in the sensitivity and reactivity of the bronchi, manifested by an attack or the status of suffocation, with constant symptoms of respiratory discomfort, against the background of a hereditary predisposition to allergic diseases. Classification The classification of bronchial asthma is as follows. 1. Stages of development of asthma: 1) biological defects in practically healthy people; 2) the state of preastma; 3) clinically pronounced asthma. 2. Clinical and pathogenetic variants: 1) atopic; 2) infectious-dependent; 3) autoimmune; 4) dishormonal; 5) neuro-psychic; 6) aspirated; 7) primary altered bronchial reactivity. 3. The severity of the course of the disease: 1) lung; 2) moderate; 3) heavy. 4. Flow phases: 1) exacerbation; 2) unstable remission; 3) stable remission (more than 2 years). 5. Complications: 1) pulmonary - atelectasis, pneumothorax, acute pulmonary insufficiency; 2) extrapulmonary - cor pulmonale, heart failure. 6. By etiology: 1) atopic (exogenous, allergic, immunological); 2) non-atopic (endogenous, non-immunological). Clinical criteria for the degree of BA are given in Table 2. Table 2 Clinical criteria for assessing the severity of asthma
asthmatic status Asthmatic status is a non-stopping attack of bronchial asthma, characterized by acute obstructive respiratory failure during the day. The main distinguishing features of status asthmaticus are the lack of effect of conventional bronchodilatory therapy and an unproductive debilitating cough. The classification of status asthmaticus is shown in Table 3. Table 3 Classification of status asthmaticus (Sorokina T. A., 1987)
AS is characterized by severe shortness of breath of an expiratory nature with the participation of the auxiliary muscles of the chest and anterior abdominal wall in the act of breathing, accompanied by a change in the color of the skin - pallor, hyperemia, cyanosis. The skin may be dry and hot or cold and damp. Tachypnea is characteristic, the respiratory rate is usually more than 30 per 1 min. Auscultatory listening to the musical sound associated with the passage of air through the narrowed bronchioles. With the progression of the process, the well-known phenomenon of "silent zones" of the lungs occurs, which indicates broncho-obstruction of this region of the lungs. Characterized by tachycardia, increased blood pressure and cardiac output (MOS). Decreased systolic blood pressure during inspiration. Dehydration and hypovolemia develop. Fluid loss occurs mainly through the respiratory tract and skin. The volume of circulating blood (CBV) is usually reduced by an average of 10% and very rarely increased. Significantly increase blood viscosity and hematocrit to 0,50-0,60, which creates a real threat of pulmonary thromboembolism and requires the appointment of heparin. The concentration of proteins is increased, general dehydration is manifested by thirst, dryness of the tongue, increased plasma osmolality, and oliguria. Central venous pressure (CVP) is reduced to 2-5 cm of water. Art. Hypovolemia predisposes to collapse, which is especially important when transferring patients to mechanical ventilation. Initially, there is excitement, then mental disorders and "respiratory panic", which is associated with a feeling of lack of air. In the future, irritability, confusion, lethargy (up to stupor and coma) sets in. Respiratory acidosis develops. Emergency treatment of status asthmaticus Oxygen therapy. Moistened oxygen is inhaled2 through nasal catheters or through a mask at a rate of 1-2 l / min. Adrenaline stimulates a1-, b1- and b2-adrenergic receptors, dilates the bronchi and reduces airway resistance. It is administered subcutaneously: with a body weight of less than 60 kg - 0,3 ml, with a weight of 60 to 80 kg - 0,4 ml, with a weight of more than 80 kg - 0,5 ml. Eufillin inhibits phosphodiesterase, which contributes to the accumulation of cAMP and the removal of bronchospasm. When prescribing aminophylline, contraindications should be taken into account, which include smoking and childhood, heart failure and acute coronary syndrome, chronic diseases of the lungs, liver and kidneys. With AS, the loading dose of aminophylline is 3-6 mg/kg, it is administered intravenously over 20 minutes. Then, a maintenance drip infusion of the drug is carried out at the rate of 0,6 mg/kg per 1 hour for a patient without concomitant pathology, 0,8 mg/kg per 1 hour for a smoker, 0,2 mg/kg per 1 hour for congestive heart failure, pneumonia , diseases of the liver and kidneys, 0,4 mg / kg per 1 hour for severe chronic lung diseases. The effect of corticosteroid therapy is associated with the suppression of airway inflammation and increased sensitivity to b-adrenergic drugs. The more severe the AS, the greater the indication for immediate corticosteroid therapy. A high dose of corticosteroids should be administered initially. The minimum dose is 30 mg of prednisolone or 100 mg of hydrocortisone, or 4 mg of dexamethasone (celeston). If therapy is ineffective, the dose is increased. At least every 6 hours, appropriate equivalent doses of these drugs are administered. Most patients are shown inhalation therapy with b-adrenergic agonists; (fenoterol, alupent, salbutamol). Exceptions are cases of drug overdose of sympathomimetics. If the ongoing therapy does not give an effect, intravenous administration of b-adrenergic agonists, such as isoproterenol, diluted in a 5% glucose solution, is indicated. Contraindications are heart disease (coronary cardiosclerosis, myocardial infarction), severe tachycardia and symptoms of tachyphylaxis, old age. The rate of administration of isoproterenol is 0,1 μg / kg per 1 min until the onset of tachycardia (HR 130 per 1 min or more). Infusion therapy is the most important component of the treatment of AS, aimed at replenishing fluid deficiency and eliminating hypovolemia, the total volume of infusion therapy is 3-5 liters per day. Hydration is carried out by introducing solutions containing a sufficient amount of free water (glucose solutions), as well as hypo- and isotonic electrolyte solutions containing sodium and chlorine. Indicators of adequate hydration are the cessation of thirst, a wet tongue, the restoration of normal diuresis, improved sputum evacuation, and a decrease in hematocrit to 0,30-0,40. Halothane anesthesia can be used in the treatment of a severe asthma attack that is not amenable to conventional therapy. Artificial ventilation of the lungs. Indications for the transfer of patients with AS to mechanical ventilation should be very strict, since in this state it often causes complications and is characterized by high mortality. At the same time, mechanical ventilation, if it is carried out according to strict indications, is the only method that can prevent further progression of hypoxia and hypercapnia. Indications for IVL: 1) steady progression of AS, despite intensive therapy; 2) increase in pCO2 and hypoxemia, confirmed by a series of tests; 3) progression of CNS symptoms and coma; 4) increasing fatigue and exhaustion. Mucolytics and expectorants are divided into two groups. 1. Proteolytic enzymes (trypsin, chymotrypsin) act by breaking the peptide bonds of glycoproteins, reducing the viscosity and elasticity of sputum. They are effective in mucous and purulent sputum, having an anti-inflammatory effect, but can cause hemoptysis and allergic reactions. 2. Cysteine derivatives stimulate secretory activity in the ciliated epithelium of the tracheobronchial tree (mukosolvan, mukomist), are used as an aerosol of a 20% solution of 2-3 ml 2-3 times a day. Lecture No. 6. Emergency conditions in cardiology 1. Myocardial infarction Myocardial infarction is a discrepancy between myocardial oxygen demand and its delivery, resulting in limited necrosis of the heart muscle. The most common cause is a thrombus, less often an embolus, less often a spasm of the coronary arteries. Thrombosis is most often observed against the background of atherosclerotic damage to the coronary arteries. In the presence of atheromatous plaques, a swirling of the blood flow occurs. Atherosclerotic lesions develop as a result of impaired lipid metabolism, blood clotting increases, which is associated with a decrease in the activity of mast cells that produce heparin. Increased blood clotting and turbulence contribute to the formation of blood clots. Disintegration of atheromatous plaques, hemorrhages in them can lead to the formation of blood clots. Predisposing factors are male gender, age over 50, obesity, heredity, psycho-emotional stress, hard work. Clinic and diagnostics Classically, myocardial infarction begins with increasing pain behind the sternum, which is burning and pressing in nature. Characterized by extensive irradiation of pain in the arms (more often in the left), back, abdomen, head, under the left shoulder blade, in the left lower jaw, etc. Patients are restless, anxious, sometimes they note a feeling of fear of death. There are signs of heart and vascular insufficiency - cold extremities, clammy sweat, etc. The pain syndrome is prolonged, and is not relieved by nitroglycerin for 30 minutes or more. There are various disorders of the heart rhythm, a drop in blood pressure or its rise. Patients subjectively note the feeling of lack of air. The above signs are typical for period I - painful or ischemic, the duration of which ranges from several hours to 2 days. Objectively, an increase in blood pressure (then a decrease); increased heart rate or rhythm disturbance; on auscultation, an abnormal IV tone is heard; heart sounds are muffled; on the aorta accent II tone; there are practically no biochemical changes in the blood, characteristic signs on the ECG. The second period is acute (feverish, inflammatory), characterized by the occurrence of necrosis of the heart muscle at the site of ischemia. The pain usually goes away. The duration of the acute period is up to 2 weeks. The patient's state of health gradually improves, but general weakness, malaise, and tachycardia persist. Heart sounds are muffled. An increase in body temperature due to the inflammatory process in the myocardium, usually small, up to 38 ° C, usually appears on the 3rd day of the disease. By the end of the first week, the temperature usually returns to normal. When examining blood, they find: leukocytosis, moderate, neutrophilic (10-15 thousand) with a shift to rods: eosinophils are absent or eosinopenia; gradual acceleration of ESR; C-reactive protein appears; increased transaminase activity; increased activity of lactate dehydrogenase, creatine phosphokinase and other markers of infarction. Cardiospecific are CPK-MB fraction and cardiac troponin. The third period (subacute, or scarring) lasts 4-6 weeks. Characteristic for it is the normalization of blood parameters (enzymes), body temperature normalizes, all other signs of an acute process disappear: the ECG changes, a connective tissue scar develops at the site of necrosis. The fourth period (rehabilitation period, recovery) lasts from 6 months to 1 year. There are no clinical signs. During this period, compensatory hypertrophy of intact myocardial muscle fibers occurs, and other compensatory mechanisms develop. There is a gradual restoration of myocardial function. But the pathological Q wave persists on the ECG. But we should not forget about the presence of atypical forms of myocardial infarction, which are often found in clinical practice. These include the following. 1. The abdominal form proceeds according to the type of pathology of the gastrointestinal tract with pain in the epigastric region, under the xiphoid process, in the abdomen, accompanied by nausea, vomiting. More often this form (abdominal) of myocardial infarction occurs with infarction of the posterior wall of the left ventricle. In general, the option is rare. ECG leads II, III, and VL. 2. The asthmatic form is characterized by signs of cardiac asthma and provokes pulmonary edema as an outcome. Pain may be absent. The asthmatic form occurs more often in older people with cardiosclerosis or in recurrent infarction, or in very large infarcts. There is shortness of breath, suffocation, cough. Auscultatory in the lungs - moist fine bubbling rales. 3. Brain form, or cerebral. At the same time, in the foreground, symptoms of cerebrovascular accident by the type of stroke with loss of consciousness are more common in older people with cerebral vascular sclerosis. There is dizziness, nausea, vomiting, focal neurological symptoms. 4. Silent, or painless, form is an accidental finding during medical examination. Of the clinical manifestations: suddenly it became "ill", there was a sharp weakness, sticky sweat, then everything, except for weakness, disappears. This situation is typical for a heart attack in old age and with repeated myocardial infarctions. An unmotivated decrease in exercise tolerance develops. 5. Arrhythmic form: the main symptom is paroxysmal - tachycardia, pain may be absent. It begins with a sign of ventricular or supraventricular tachycardia, AV block II-III degree, acute blockade of the legs of the atrioventricular bundle. Morgagni-Adams-Stokes attacks often occur in the debut. In most cases, cardiac arrhythmias are complicated by hypotension, arrhythmogenic shock, and acute heart failure. Signs of myocardial infarction on the ECG are as follows: 1) with penetrating myocardial infarction or transmural (i.e., the necrosis zone extends from the pericardium to the endocardium): displacement of the ST segment above the isoline, the shape is convex upward - like a “cat’s back”; fusion of the T wave with the ST segments on days 1-3; deep and wide Q wave is the main sign; decrease in the size of the R wave, sometimes QS form; characteristic discordant changes - opposite shifts of ST and T (for example, in the 1st and 2nd standard leads compared to the 3rd standard lead); on average, from the 3rd day, a characteristic reverse dynamics of ECG changes is observed: the ST segment approaches the isoline, a uniform deep T appears. The Q wave also undergoes reverse dynamics, but the altered Q and deep T can persist for life; 2) with intramural or non-transmural myocardial infarction: there is no deep Q wave, the ST segment displacement can be not only up, but also down. The main criteria for the diagnosis of myocardial infarction: 1) clinical signs; 2) electrocardiographic signs; 3) biochemical signs. Complications: cardiac arrhythmias, atrioventricular conduction disturbances, acute left ventricular failure: pulmonary edema, cardiac asthma, cardiogenic shock, gastrointestinal disorders (paresis of the stomach and intestines, gastric bleeding), pericarditis, parietal thromboendocarditis, myocardial ruptures, acute and chronic heart aneurysm, syndrome Dressler, thromboembolic complications, postinfarction angina pectoris. Treatment Treatment is aimed at preventing complications, limiting the infarct zone, pain relief and correction of hypoxia. Removal of a pain syndrome: begin with reception of nitrates. With severe hypotension, neuroleptanalgesia is performed - fentanyl 1-2 ml intravenously on glucose, droperidol 0,25% 2 ml per 40 ml 5% glucose solution. With an incomplete effect, morphine 1% 1,0 is re-introduced after an hour subcutaneously or intravenously by stream; omnopon 2% - 1,0 subcutaneously or intravenously; promedol 1% - 1,0 subcutaneously. To enhance the analgesic effect, relieve anxiety, anxiety, arousal, apply: analgin 50% - 2,0 intramuscularly or intravenously; diphenhydramine 1% - 1,0 intramuscularly (sedative effect) + chlorpromazine 2,5% - 1,0 intramuscularly, intravenously (drug potentiation). To limit the zone of necrosis, anticoagulants are used (heparin 5 thousand units - 1 ml bolus followed by intravenous administration of an infusion pump 1 thousand units per hour), thrombolytics (fibrinolysin 6 thousand units intravenously drip; streptase 250 thousand in saline intravenously drip) and antiplatelet agents (aspirin, cardiomagnyl, thrombo-ACS, Plavix). Prevention and treatment of arrhythmias. 1. Polarizing mixture, which promotes the penetration of potassium into the cells. 2. Lidocaine is the drug of choice, more effective for ventricular arrhythmias 80-100 mg bolus. 3. Cordarone or amiodarone 450 mg intravenously in saline. Given that the pumping function of the heart suffers, the appointment of b-blockers (egilok 12,5-25 mg) is indicated to enhance myocardial contractility. In the presence of edema in the lower extremities or moist rales in the lungs, diuretics are used (Lasix at a dose of 40-80 mg). Great emphasis falls on blood pressure, which must either be increased with hypotension (dopamine) or reduced (isoket intravenous drip, antihypertensive drugs - enalapril). To eliminate hypoxia, oxygen therapy is carried out using humidified oxygen through a mask or nasal catheters. 2. Cardiogenic shock Cardiogenic shock is a critical circulatory disorder with arterial hypotension and signs of acute deterioration of blood circulation in organs and tissues. Clinic and diagnostics The main diagnostic sign is a significant decrease in systolic blood pressure, which is below 90 mm Hg. Art. The difference between systolic and diastolic pressure (pulse pressure) is 20 mm Hg. Art. or getting even smaller. In addition, a clinic of a sharp deterioration in the perfusion of organs and tissues is developing: 1) impaired consciousness from mild lethargy to psychosis or coma, focal neurological symptoms may appear; 2) diuresis less than 20 ml/h. Symptoms of deterioration of peripheral circulation: pale cyanotic, marbled, brick, moist skin; collapsed peripheral veins, a sharp decrease in the temperature of the skin of the hands and feet; decrease in blood flow. The value of the CVP can be different. Normal indicators of CVP are 5-8 cm of water. Art.; indicator below 5 cm of water. Art. indicates hypovolemia and low blood pressure, and above 8 cm of water. Art. indicates right ventricular failure. Diagnosis of cardiogenic shock is usually not difficult. It is more difficult to determine its type and leading pathophysiological mechanisms. First of all, it is necessary to distinguish true (contractile) cardiogenic shock from arrhythmic, reflex (painful), medical shock due to right ventricular failure or slowly ongoing myocardial rupture. When conducting intensive care for a patient with shock, it is important to exclude causes of low blood pressure, such as hypovolemia, cardiac tamponade, tension pneumothorax, thromboembolic complications, and not to miss internal bleeding, for example, with stress erosions or ulcers of the gastrointestinal tract. Treatment Oxygen therapy with humidified oxygen through a mask or nasal catheters is indicated. Anticoagulants are administered as a bolus at a dose of 10 IU, followed by intravenous administration of an infusion pump at 000 IU per hour. It is necessary to administer analgesics: morphine 1000% 1 ml subcutaneously or intravenously by bolus; analgin 1,0% 50 ml intramuscularly, intravenously. Vascular tonics: Cordiamin 1-4 ml intravenously; mezaton 1% 1,0 g subcutaneously, intravenously, in saline; norepinephrine 0,2% 1,0 intravenously. True cardiogenic shock is treated as follows. To increase the contractile activity of the myocardium, the following is used: strophanthin 0,05% 0,5-0,75 g intravenously slowly per 20,0 isotonic solution, korglukon 0,01 g intravenously, also in an isotonic solution or in a polarizing mixture, glucagon 2- 4 mg intravenously drip on a polarizing solution. Normalization of blood pressure: norepinephrine 0,2% 2-4 ml per 1 liter of 5% glucose solution or isotonic solution. BP is maintained at 100 mm Hg. Art., mezaton 1% 1,0 g intravenously; cordiamine 2-4 ml, dopamine 200 mg in 400 ml of rheopolyglucin or 5% glucose. With an unstable effect from the above drugs, hydrocortisone 200 mg, prednisolone 90-120 mg are used. Normalization of the rheological properties of blood (since microvascular thrombi are necessarily formed, microcirculation is disturbed). Elimination of hypovolemia, as there is sweating of the liquid part of the blood: reopoliglyukin, polyglukin - in a volume of up to 100 ml at a rate of 50,0 ml per minute. Correction of acid-base balance (fight against acidosis): sodium bicarbonate 5% to 200,0 ml. Re-introduction of painkillers. Restoration of rhythm and conduction disturbances. But it is always necessary to control the CVP, which allows the resuscitator to determine the acceptable infusion therapy. Patients with cardiogenic shock should not be loaded with water. The higher the CVP, the less infusion therapy. 3. Hypertensive crisis A hypertensive crisis is a sudden increase in blood pressure to a level that is usually not characteristic of this patient, leading to acute regional circulatory disorders and damage to target organs (heart, brain, kidneys, intestines). External factors provoking a crisis can be: 1) psycho-emotional stress; 2) meteorological influences; 3) excessive consumption of table salt. In spring and autumn, crises occur more often than in winter and summer. Crises can also occur against the background of an exacerbation of a number of chronic diseases. MS Kushakovsky (1982) distinguishes the following variants of hypertensive crises: neurovegetative, water-salt, convulsive (encephalopathy). Clinic The clinical symptoms of the crisis are manifested by tinnitus, flashing flies before the eyes, bursting headache in the occipital region, aggravated by bending over, straining, coughing, nausea, vomiting, and heart rhythm disturbances. During a crisis, dangerous violations of the cerebral coronary, less often renal and abdominal circulation occur, which leads to stroke, myocardial infarction and other serious complications. ECG reveals left ventricular hypertrophy. Chest x-ray shows an enlarged heart, aortic deformity in the form of the number "3", usury of the ribs as a result of increased collateral blood flow through the intercostal arteries. Aortography confirms the diagnosis. The neurovegetative form of the crisis is characterized by a sudden onset, excitation, hyperemia and moisture of the skin, tachycardia, frequent profuse urination, a predominant increase in systolic pressure with an increase in pulse amplitude. Such crises are otherwise called adrenal, or type I crises. Type I crises usually have a relatively benign course, although they can lead to paroxysmal arrhythmias or angina pectoris, and in severe cases, myocardial infarction. With the water-salt form of the crisis, the condition worsens gradually, drowsiness, weakness, lethargy, disorientation, pallor and puffiness of the face, and swelling are noted. Systolic and diastolic pressure increase evenly or with a predominance of the latter and a decrease in pulse pressure. Such crises are called type II crises. Crises of type II, as a rule, are severe and can be complicated by myocardial infarction, stroke, acute left ventricular failure. It is necessary to highlight hypertensive crises that develop as a result of an abrupt cessation of permanent antihypertensive therapy, in particular, taking b-blockers, nifedipine, sympatholytics, and especially clonidine. Treatment Treatment of a hypertensive crisis consists in an urgent decrease in blood pressure to a normal level, necessary to prevent or limit damage to target organs in hypertension, to prevent complications up to death in the most severe cases, or permanent disability in the development of stroke, myocardial infarction. In the neurovegetative form of the crisis, intravenous jet, slow administration of 0,1 mg of clonidine or repeated intravenous infusions of 50 mg of labetalol are usually used. The hypotensive effect of clonidine can be enhanced by sublingual administration of 10 mg of nifedipine. In extremely severe cases, sodium nitroprusside is injected intravenously, and in its absence, intravenous drip or very slowly fractionally - up to 50 mg of pentamine. The main dangers and complications of antihypertensive therapy: 1) arterial hypotension; 2) violation of cerebral circulation (hemorrhagic or ischemic stroke, encephalopathy); 3) pulmonary edema; 4) angina pectoris, myocardial infarction; 5) tachycardia. Life-threatening hypertensive crises are grounds for immediate intensive care. Types of hypertensive crises. 1. Convulsive form of hypertensive crisis (acute severe hypertensive encephalopathy). 2. Crisis with pheochromocytoma. 3. Acute arterial hypertension in life-threatening diseases and conditions (acute coronary syndrome, acute myocardial infarction, dissecting aortic aneurysm, internal bleeding). 4. Hypertensive crisis complicated by pulmonary edema or hemorrhagic stroke. A convulsive form of a hypertensive crisis (acute severe hypertensive encephalopathy) develops in a malignant form of hypertension or secondary arterial hypertension, for example, in late toxicosis of pregnant women or acute glomerulonephritis. Crises begin with a severe throbbing, arching headache, psychomotor agitation, repeated vomiting that does not bring relief, visual disturbances; loss of consciousness quickly occurs and clonic-tonic convulsions appear. In patients with recent arterial hypertension (with acute glomerulonephritis, toxicosis of pregnant women), a convulsive hypertensive crisis may develop with a relatively small increase in blood pressure. In the convulsive form of the crisis, emergency care is aimed at eliminating the convulsive syndrome and an emergency lowering of blood pressure. The convulsive syndrome is eliminated by intravenous administration of diazepam. Additionally, 10 ml of a 25% solution of magnesium sulfate can be administered intravenously by drip or slow stream, or intramuscularly. Sodium nitroprusside, labetalol, diazoxide are used to urgently lower blood pressure. To combat cerebral edema, intravenous jet administration of lasix is indicated. With eclampsia, especially with intravenous administration, magnesium sulfate is still widely used, which is administered at a dose of 4 g per 100 ml of a 5% glucose solution. Then, if necessary, a drip injection of the drug is carried out, or instead of the subsequent drip injection of magnesium sulfate, 20 ml of a 25% solution of magnesium sulfate can be injected deep intramuscularly. Intravenous magnesium sulfate should be avoided if the pregnant woman is being treated with calcium antagonists (a sharp drop in blood pressure is dangerous). Perhaps with eclampsia and intravenous administration of chlorpromazine (100-250 mg), diazoxide (300 mg). Seduxen (diazepam) is administered intravenously slowly (20-30 mg), and then drip (300 mg in 500 ml of 5% glucose solution). A crisis in pheochromocytoma is manifested by a sudden, very rapid and sharp increase in blood pressure, mainly systolic, and an increase in pulse pressure, accompanied by pale skin, cold sweat, palpitations, pain in the heart and epigastric region, nausea, vomiting, throbbing headache, dizziness. During a crisis, an increase in body temperature, visual and hearing disorders are possible. Characterized by a decrease in blood pressure after the transition to a vertical position. Emergency care in cases of crisis with pheochromocytoma begins with raising the head end of the bed to an angle of 45 °, which causes a decrease in blood pressure. For emergency antihypertensive therapy, the drug of choice is phentolamine, which is administered intravenously in a stream of 5 ml every 5 minutes. For the same purpose, intravenous injection of labetalol 50 ml every 5 minutes or drip infusion of 30 ml of sodium nitroprusside in 300 ml of 5% glucose solution is used. As an additional drug, droperidol (5-10 ml IV) may be useful. To suppress tachycardia, propranolol is prescribed at a dose of 20-40 mg. In acute myocardial infarction (especially often noted in its anterior localization), when during a crisis the load on the myocardium increases, myocardial oxygen demand increases, it is first necessary to stop a severe pain attack with the help of modern painkillers (including narcotic analgesics) and administer sedatives which can significantly lower blood pressure. If significant hypertension persists and at the same time the tone of the sympathetic nervous system is increased, then b-blockers (propranolol, metoprolol, esmolol) are administered intravenously, which, along with the hypotensive effect, can limit the area of peri-infarction myocardial ischemia. Often resort to intravenous administration of nitroglycerin to cause a decrease in pre- and afterload. This allows you to control the level of blood pressure. However, the appointment of sodium nitroprusside should be avoided, which in these cases can increase myocardial ischemia, apparently due to the phenomenon of "coronary steal" (reduction of coronary collateral blood flow to the ischemic zone). If hypertension persists after the acute stage of myocardial infarction, treatment with the main antihypertensive agents is started, taking into account indications and contraindications. However, in recent years, in connection with data on secondary prevention, b-blockers and ACE inhibitors are most often preferred, which, in the absence of contraindications, are tried to be prescribed from an early period. With the development of acute heart failure, the hypertensive crisis is stopped by intravenous administration of nitroglycerin (contraindicated in severe mitral stenosis); prescribe vasodilators (although tolerance often develops to them), IFKA. With persistent hypertension in cases of severe heart failure, a combination with diuretics is resorted to, and even small doses of a thiazide diuretic together with a potassium-sparing diuretic (triamterene or amiloride) can not only normalize blood pressure, but also prevent the occurrence of arrhythmias caused by potassium and magnesium deficiency in such patients. (Metelitsa V.I., 1996). In hemorrhagic stroke or subarachnoid hemorrhage, blood pressure should be reduced especially carefully, with the help of drugs, the hypotensive effect of which can be easily controlled (sodium nitroprusside), and to a level higher than usual (working). Any decrease in blood pressure, accompanied by a deterioration in neurological status, should be considered excessive. In case of pulmonary edema, nitroglycerin or sodium nitroprusside or pentamine, as well as lasix, are prescribed intravenously to urgently lower blood pressure. With a dissecting aortic aneurysm or rupture, the following drugs are used intravenously to regulate blood pressure and prepare for surgical treatment: sodium nitroprusside, loop diuretics (furosemide), nifedipine, propranolol (along with sodium nitroprusside), methyldopa, reserpine (as an additional agent). In crises caused by an increase in cardiac output, tachycardia and a predominant increase in systolic and pulse pressure are often observed, a good effect in these cases is the intravenous administration of anaprilin, and then, if necessary, furosemide. 4. Life-threatening arrhythmias Arrhythmia An arrhythmia is a heart rhythm other than sinus. A normal heart rhythm has the following characteristics: 1) heart rate from 60 to 120 per minute; 2) the pacemaker is the sinus node, evidence of which is a positive P wave preceding the QRS complex in standard lead II and negative in AVR; 3) RR intervals differ by no more than 0,01 s; 4) the actual indicators, reflecting the size of the intervals and teeth in the norm. All changes on the ECG are carried out in the II standard lead. Arrhythmia classification 1. Violation of the formation of impulses: 1) in the sinus node: a) sinus tachycardia; b) sinus bradycardia; c) sinus arrhythmia; d) sick sinus syndrome (SSS); 2) ectopic arrhythmias: a) extrasystole; b) paroxysmal tachycardia; c) atrial fibrillation and flutter; d) flicker and flutter of the ventricles. 2. Violation of impulse conduction: 1) additional pathways (Kent bundles); 2) heart block: a) atrial (intra-atrial); b) atrioventricular; c) intraventricular. Mechanisms of arrhythmias A decrease in the resting potential, the excitability threshold occurs only on the basis of a deficiency of cellular potassium, the ratio "plasma - cell" (normally 80 meq of potassium is in the cell and 5 meq in plasma). Asymmetry of the electrophysiological-metabolic focus of the myocardium due to ischemia, inflammation, reperfusion during thrombolysis. Electrophysiological weakness of the superior pacemaker. Congenital accessory conduction pathways. Paroxysmal supraventricular tachycardia Paroxysmal supraventricular tachycardia is a sudden attack of heartbeat with a frequency of 150-250 beats per minute. There are 3 forms: 1) atrial; 2) nodal; 3) ventricular. The etiology of supraventricular paroxysmal tachycardia is more often associated with an increase in the activity of the sympathetic nervous system. It is clinically manifested by a sudden attack of the heartbeat, the vessels of the neck pulsate, cardiac activity switches to a different rhythm. The duration of the attack is from several minutes to several days. The number of heartbeats in the ventricular form is usually in the range of 150-180 beats per minute, with supraventricular forms - 180-240 beats per minute. During an attack, a pendulum-like rhythm is characteristic auscultatory, there is no difference between I and II tone. It increases myocardial oxygen demand and can provoke an attack of acute coronary insufficiency. ECG signs 1. QRS complexes are not changed. 2. In the supraventricular form, the P wave merges with T. Treatment begins with intravenous administration of cordarone at a dose of 300 mg or novocainamide up to 1 g, and then adenosine 1 ml - 1% (10 mg) bolus. Calcium antagonists verapamil (Isoptin) are used intravenously as a bolus at a dose of 2,5-5 mg over 2-4 minutes. But it is used for narrow QRS complexes, and for wide ones it can fibrillate. It is possible to take b-blockers (propranolol 20-40 mg sublingually). Paroxysm of atrial fibrillation Paroxysm of atrial fibrillation is characterized by the absence of atrial complexes, and instead of an isoline, sawtooth waves of atrial flutter are detected, which are most distinct in leads II, III, and VF with a frequency of 250-400 beats per minute. Or there are no atrial complexes, flicker waves, large- or small-wave oscillations of the isoline are detected, the frequency of atrial waves is 350-600 beats per minute. Clinic. The pulse is arrhythmic with waves of different filling, the presence of a pulse deficit (the difference between heart rate and pulse); different intervals and different volume of heart sounds during auscultation. Treatment. In case of paroxysm, it begins with the introduction of digoxin 0,25 mg (1 ml of 0,025%) per 20 ml of physiological saline as an intravenous bolus. To achieve the desired effect of saturation with glycosides, a dose of 1,5 mg of digoxin per day or 3 days is recommended. With uncomplicated paroxysm, the drug of choice is novocainamide, administered intravenously slowly at a dose of up to 2000 ml over 30 minutes (10 ml of a 10% solution) with constant monitoring of blood pressure, heart rate, ECG. Atrial flutter is treated with electrical impulse therapy. Paroxysmal ventricular tachycardia Paroxysmal ventricular tachycardia is characterized by the detection of 3 or more consecutive wide (more than 0,12 s) QRS complexes with a frequency of 100-250 beats per minute with a discordant shift of the ST segment and the T wave in the direction opposite to the main wave of the QRS complex. Pirouette, or bidirectional, fusiform ventricular tachycardia occurs when the QT interval is lengthened. In this case, an irregular rhythm is recorded with a heart rate of 150-250 beats per 1 min with wide polymorphic deformed QRS complexes. Treatment. In conditions of hypodynamia of blood circulation, electrical impulse therapy is required, after which maintenance therapy is carried out with lidocaine drip. In conditions of stable hemodynamics, the drug of choice is lidocaine, intravenous bolus 1-2 mg/kg (80-100 mg) for 3-5 minutes, followed by drip infusion during the day at 4 mg/min. Ventricular extrasystole Ventricular extrasystole is the occurrence of an extraordinary wide deformed QRS complex, discordant ST and T shift, a complete compensatory pause (the interval between the pre- and post-extrasystolic P wave is equal to twice the normal RR interval). The drug of choice is lidocaine, which is administered according to the above scheme. Perhaps the use of cordarone at a dose of 300-450 mg intravenously drip. Violation of AV conduction with the development of syncope (Morgagni-Adams-Stokes syndrome) When conduction is disturbed, various types of heart blocks occur, there is a slowdown or complete cessation of the conduction of the impulse through the conduction system of the heart. Sinoauricular blockade is characterized by dysfunction of T cells and impaired conduction of impulses from the sinus node to the atria. There are 3 degrees. I degree - slowing down the impulse. On the ECG - prolongation of the PQ interval for more than 0,20 s. Prolapse of the QRS complex. The RR interval is stable. II degree - loss of part of the impulses, incomplete conduction. Mobitz type I - as the impulses are carried out, the PQ interval gradually lengthens until the complete loss of the pulse wave. QRS is not changed. At the site of the QRS prolapse, the greatest distance is RR. Prognostically, this type is relatively favorable. Mobitz type II with a constant PQ interval and an unchanged QRS complex. At the same time, not all impulses reach the ventricles - in some cases, every second impulse is carried out, in others - every third, etc., i.e., there is a periodic prolapse of the QRS complex 3: 2, 4: 3, 5: 6, etc. d. III degree - complete blockade of conduction. At the same time, the conduction of impulses to the ventricles is completely stopped, and a heterotopic focus of idioventricular rhythm is born in the ventricles, and the lower the automatism, the more difficult the clinic. Complete dissociation is observed: the atrial rhythm is close to normal, and the ventricles have their own frequency - 40 beats per minute or less. The latter depends on the level of damage: if the AV node suffers, 40-50 beats per 1 minute, if the leg of the bundle of His - 20 beats per 1 minute or less. The level of damage is also indicated by the degree of deformation of the QRS complex. The heart sounds are weakened, periodically there is a "cannon" I tone, when the systole of the atria and ventricles almost coincide in time. May be III additional tone. Systolic ejection murmurs may appear at the base of the heart. Often a pulsation of the veins associated with atrial contraction is found, especially distinct with Strazhesko's cannon tone. Clinic. Failing of the heart, if one impulse falls out. Vertigo if several impulses fall out. Morgagni-Adams-Stokes syndrome (loss of consciousness), if 6-8 complexes fall out. Treatment. To restore an adequate rhythm, atropine is administered at a dose of 0,5-1 mg to 3 mg. Every 3 minutes, 1 mg to a total dose of 0,4 mg/kg. Calcium antagonists - isoptin 0,04 mg/kg. With frequent loss of consciousness, the patient is transferred to permanent electropulse therapy. But more often pacing has to be done "on demand". Lecture number 7. Acute renal failure Acute renal failure (ARF) is a complication of a number of renal and extrarenal diseases characterized by a sharp deterioration or cessation of kidney function and manifested by the following symptom complex: oligoanuria, azotemia, hyperhydration, impaired CBS and water and electrolyte balance. The forms of OOP include: 1) prerenal (hemodynamic); 2) renal (parenchymal); 3) postrenal (obstructive); 4) arenal. Etiology Reasons for the development of prerenal acute renal failure. 1. Decreased cardiac output (cardiogenic shock, paroxysmal arrhythmia, cardiac tamponade, pulmonary embolism, congestive heart failure). 2. Reduced vascular tone (sepsis, infectious-toxic shock, anaphylactic shock, overdose of antihypertensive drugs). 3. Decreased effective intravascular volume (blood loss, plasma loss, dehydration - loss of 7-10% of body weight, profuse vomiting, diarrhea, polyuria, hypovolemia with nephropathy in pregnancy, nephrotic syndrome, peritonitis, liver cirrhosis). 4. Violation of intrarenal hemodynamics (taking NSAIDs, ACE inhibitors, radiopaque drugs, sandimmune). 5. Water poisoning - hyperhydration (uncontrolled production of ADH in malignant tumors, inflammatory diseases of the central nervous system, drug overdose - drugs, barbiturates, antidiabetic sulfanilamide drugs, indomethacin, amitriptyline, cyclophosphamide). Reasons for the development of renal acute renal failure. 1. Kidney ischemia (shock, dehydration). 2. Nephrotoxic damage due to exposure to: 1) drugs (aminoglycosides, NSAIDs, radiopaque agents, sulfonamides, phenacetin, barbiturates, cephalosporins, ampicillin, rimfapicin, sandimmune); 2) industrial nephrotoxins (salts of heavy metals: mercury, chromium, cadmium, lead, arsenic, platinum, bismuth, gold, uranium, barium); 3) household nephrotoxins (ethylene glycol, methyl alcohol, dichloroethane, carbon tetrachloride). 3. Intratubular obstruction by pigments: 1) hemoglobin (hemolysis - incompatible blood transfusion, mushroom poisoning, acetic acid, hemolytic anemia, hemolytic-uremic syndrome, thrombotic thrombocytopenic purpura); 2) urates (gout, immunosuppressive therapy for multiple myeloma and leukemia in severe physical exertion, in people not adapted to the heat); 3) myoglobin (traumatic rhabdomyolysis, non-traumatic rhabdomyolysis in coma, electrical injury, frostbite, eclampsia, alcoholic and heroin myopathy, severe hypokalemia and hypophosphatemia, carbon monoxide poisoning, salts of mercury, zinc, copper, drugs, viral myositis, overdose of statins and fibrates); 4) inflammatory processes: OTIN of medicinal and infectious genesis (AIDS, HFRS, measles, mononucleosis, leptospirosis, mycoplasmosis, rickettsiosis) acute pyelonephritis, acute glomerulonephritis; 5) necrotic papillitis (diabetes mellitus, analgesic, alcoholic nephropathy); 6) vascular pathology (vasculitis - polyarteritis nodosa, Wegener's granulomatosis, systemic scleroderma; thrombosis of arteries or veins, bilateral embolism of the renal arteries, traumatic injury). Reasons for the development of postrenal acute renal failure. 1. Pathology of the ureters: 1) obstruction (stone, blood clots, necrotic papillitis); 2) compression (tumor of the pelvic organs, retroperitoneal fibrosis). 2. Pathology of the bladder (stones, tumors, inflammatory obstruction of the bladder neck, prostate adenoma, impaired innervation in spinal cord lesions and diabetic neuropathy). 3. Urethral stricture. Classification OPN classification according to E. M. Tareev. 1. Shock kidney. 2. Toxic kidney. 3. Acute infectious kidney. 4. Vascular obstruction. 5. Urological kidney. Options for the course of acute renal failure: cyclic, recurrent and irreversible. Clinic There are five stages in the clinical course of acute renal failure. Stage I of acute renal failure is initial, it lasts from the moment the etiological factor occurs until the first signs appear. At this stage, therapeutic tactics are aimed at eliminating or mitigating the impact of the etiological factor: anti-shock therapy, replenishing the BCC, combating heart failure, alkalizing therapy for intravascular hemolysis, combating pain, treating septic conditions, etc. Along with etiological therapy, spasm of kidney vessels is eliminated under the control of hourly diuresis. The earlier diuresis stimulation is started, the better the prognosis. Stage II of acute renal failure, or oligoanuric, is characterized by dysfunction of 70% of nephrons. Urination less than 500 ml per day indicates the development of oliguria, and its decrease to 50 ml per day. and below indicates anuria. Along with a violation of the water-excreting ability of the kidneys, the concentration and nitrogen-excreting functions also suffer. In the urine, the amount of electrolytes and nitrogen sharply decreases. At this stage, the most pronounced changes in hemostasis occur. Treatment should be aimed at maintaining a constant internal environment in order to give time and opportunity for the renal epithelium to regenerate. A state of hyperhydration develops due to the loss of electrolytes during vomiting and diarrhea. Therefore, it is necessary to stimulate diuresis, but only under the control of central venous pressure. Improves renal blood flow. Since it is necessary to strictly control diuresis, catheterization of the bladder is performed. Impaired nitrogen excretion function of the kidneys leads to azotemia, therefore, to maximally prevent the breakdown of proteins in the body, it is necessary to introduce a sufficient amount of carbohydrates (at least 5 g/kg per day.). Fructose and glucose are introduced, adding xylitol (sorbitol) to them in proportions of 2: 1: 1, and if there is no fructose, then 3 parts of glucose and 1 part of sorbitol. If the course is severe and cannot be treated, then hemodialysis sessions are performed. If the etiological factor is removed, then after 5-7 days of treatment, diuresis begins to increase. The maximum duration of this stage is up to 2 weeks. III stage of acute renal failure - early polyuric. It is characterized by a progressive increase in diuresis (by 200-300 ml per day) up to 3 liters. The nitrogen excretion and concentration functions of the kidneys have not yet fully recovered, but the concentration of potassium, magnesium, and phosphates is gradually normalizing. Intensive therapy in the early polyuric stage should include the same measures as in the previous one, except for the stimulation of diuresis. Often, hemodialysis is required. There is a high risk of dehydration. IV stage of acute renal failure - late polyuria. The daily increase in urine reaches 500-1000 ml, and diuresis can reach 8-10 liters per day or more. In the kidneys, ion exchange processes begin to recover. Losses of potassium, magnesium, phosphorus and other electrolytes sharply increase, patients are at risk of dehydration and demineralization. Therefore, electrolytes and fluid are given intravenously at this stage. Stage V OPN, or recovery stage. The concentration function of the kidneys is restored. Diuresis begins to gradually decrease to normal (2-3 liters per day) and urine density increases (1008-1028). To determine the severity of the disease and the effectiveness of treatment, patients with acute renal failure are examined daily in the blood for indicators of CBS, the concentration of electrolytes, hemoglobin, sugar, total protein and protein fractions, urea, residual and urea nitrogen, creatinine, hematocrit, and in daily urine - density, the amount of electrolytes and nitrogen. Treatment The principles of treatment are as follows. 1. Treatment of shock: anti-shock measures, glucocorticosteroids. 2. Replenishment of BCC: polyglucin, reopoliglyukin, plasma, albumin, erythrocyte mass. 3. Treatment of infections: adequate antibiotic therapy. 4. Dehydration: isotonic, hypertonic, hypotonic solution of sodium chloride, glucose. 5. Poisoning with poisons: antidotes are introduced. 6. Urological kidney: elimination of obstruction. 7. Intratubular obstruction: continuous up to 60 hours intensive infusion alkalizing therapy (mannitol 10% solution 3-5 ml / kg / h with isotonic sodium chloride solution, sodium bicarbonate, glucose 400-600 ml / h, furosemide 30-50 mg /kg). 8. Elimination of spasm of renal vessels: eufillin 2,4% - 10 ml again after 4-6 hours, chimes 0,5% - 2-4-6 ml intravenously, trental 3-5 mg / kg per day, pentamine 0,5-1,0 mg/kg per day, benzohexonium 0,3-0,5 mg/kg per day, droperidol 0,12 mg/kg per day, dopamine 1,5-3 mg/kg. 9. Stimulation of diuresis (after stabilization of blood pressure and elimination of hypovolemia): aminofillin, mannitol, lasix. 10. Control of hourly diuresis, blood pressure, CVP. Indications for acute hemodialysis: hypercatabolic acute renal failure, lack of effect from conservative therapy in non-catabolic acute renal failure for 2-3 days, hyperkalemia more than 6-6,5 mmol/l, decompensated metabolic acidosis with a base deficiency of more than 15 mmol/l, blood creatinine more than 600 µmol /l, blood urea more than 30 mmol/l, hyperhydration with the development of pulmonary or cerebral edema. Lecture number 8. Acute liver failure Acute liver failure is a symptom complex characterized by a violation of one or more liver functions due to acute or chronic damage to its parenchyma. Etiology The causes of acute renal failure can be hepatitis viruses A, B, C, D, E, G, as well as herpes viruses, cytomegalovirus, infectious mononucleosis virus, simple and herpes zoster, Coxsackie, measles, septicemia that develops with liver abscesses and purulent cholangitis, drugs, alcohol, industrial toxins, heart failure. OPN always proceeds with multiple organ damage: the kidneys, the cardiovascular system, the lungs, the pancreas, and the brain are involved in the process. Impaired renal function manifests as acute tubular necrosis. Pulmonary complications - aspiration of gastric contents or blood, atelectasis, respiratory infections. Acute pancreatitis and pancreatic necrosis can cause death. A life-threatening disorder of homeostasis develops. Liver failure is explained by dystrophy and widespread necrobiosis of hepatocytes. Clinic and diagnostics The clinical manifestations of ARF are as follows. 1. Coagulopathy is caused by a deficiency of coagulation factors and an increase in fibrinolytic activity. It predisposes to spontaneous bleeding from the mucous membranes: gastrointestinal, uterine, nasal bleeding can be observed. Brain hemorrhages are possible. To assess the state of the hemostasis system, prothrombin time is determined. 2. Hypoglycemia is characterized by a high level of insulin in plasma, which is due to a decrease in its uptake by the liver. It leads to a rapid deterioration of the neurological status and death of patients. 3. Violations of water-electrolyte and acid-base balance. End-stage acute renal failure is characterized by hyponatremia, hypophosphatemia, hypocalcemia, and hypomagnesemia. The change in the acid-base state does not have an unambiguous direction. Respiratory alkalosis associated with stimulation of the respiratory center with toxic substances may be replaced by respiratory acidosis due to increased intracranial pressure and suppression of respiratory activity. In the development of hepatic coma as a severe course of the disease, the stages of precoma, threatening coma and coma proper are distinguished. There are also hepatocellular (endogenous) coma, resulting from massive necrosis of the parenchyma, porto-caval (bypass, shunt, exogenous), due to a significant exclusion of the liver from metabolic processes due to the presence of pronounced porto-caval anastomoses, and mixed coma, occurring mainly with cirrhosis of the liver. In the precomatous period, progressive anorexia, nausea, a decrease in the size of the liver, an increase in jaundice, hyperbilirubinemia, and an increase in the content of bile acids in the blood develop. In the future, neuropsychic disorders, slowing of thinking, depression, and sometimes euphoria increase. Characterized by instability of mood, irritability, memory is disturbed, sleep is disturbed. Tendon reflexes increase, a small tremor of the limbs is characteristic. Azotemia develops. With timely therapy, patients can get out of this state, but more often with severe irreversible changes in the liver, coma occurs. During the period of coma, excitation is possible, which is then replaced by depression (stupor) and a progressive impairment of consciousness up to its complete loss. Meningeal phenomena, pathological reflexes, motor restlessness, convulsions develop. Breathing is disturbed (such as Kussmaul, Cheyne-Stokes); the pulse is small, arrhythmic; there is hypothermia. The patient's face is haggard, the extremities are cold, a characteristic sweet liver smell comes from the mouth and skin, hemorrhagic phenomena intensify (skin hemorrhages, bleeding from the nose, gums, varicose veins of the esophagus, etc.). Acute liver failure develops quickly, within a few hours or days, and with timely therapy can be reversible. Laboratory studies: bilirubin, urea and creatinine in blood and urine, parameters of the hemostasis system, complete blood count and urine, CVP, ECG, plasma and urine osmolarity, plasma electrolytes, free plasma and urine hemoglobin, ALT, AST, alkaline phosphatase, LDH, CPK, prothrombin time. Computed tomography of the liver can reveal a decrease in its size, but most clinicians focus on clinical and laboratory data. Treatment Timely inotropic support is an essential component of intensive care. Prevention of infectious complications - the appointment of cephalosporin antibiotics in combination with antifungal drugs (amphotericin-B). Hepatoprotectors and membrane stabilizing drugs: prednisolone up to 300 mg, vitamin C 500 mg, troxevasin 5 ml, sodium etamsylate 750 mg, Essentiale 30 ml, tocopherol 4 ml intramuscularly, cytomak 35 mg, cocarboxylase 300 mg, nicotinic acid 30-40 mg, complamin 900 mg, sirepar 5-10 ml, glutamic acid 1% 400 ml, vikasol 10 ml intravenously, B vitamins. Protease inhibitors, which include cortrical 100 thousand units, trasilol 400 thousand units, antagonosan, gordox. Stimulation of diuresis: reogluman 400 ml, mannitol, lasix up to 200 mg intravenously, eufillin 240 mg. To correct coagulopathy, intravenous administration of vitamin K (10 mg per day for 3 days) is used. The effect occurs after 3 hours. In this case, the elimination of hypoprothrombinemia associated with impaired absorption of vitamin K, resulting from a deficiency of bile acids. In case of bleeding or suspected invasive procedures (vascular catheterization, peritoneal dialysis), platelet mass or fresh frozen plasma is administered intravenously. Cerebral edema is a common cause of death. Mannitol is administered at the rate of 1 g/kg of body weight. In patients with renal insufficiency, mannitol is prescribed in combination with ultrafiltration to avoid hyperosmolarity and overhydration. With the development of hepatic coma, potassium chloride is prescribed (0,4-0,5% solution in a 5% glucose solution with a volume of 500 ml intravenously drip) or sodium bicarbonate solution (with metabolic acidosis); Patients breathe humidified oxygen through a nasal catheter. With a decrease in both arterial and venous pressure, polyglucin and albumin are administered intravenously. In the presence of massive bleeding, appropriate measures are taken to stop them, one-group blood is transfused, and drugs that contain blood clotting factors are administered. With significant signs of disseminated intravascular coagulation, heparin is administered intravenously at a dose of 10-000 IU bolus. In case of renal failure, peritoneal hemodialysis and plasmapheresis are performed, which give a good result, but before carrying out these manipulations, the introduction of heparin is contraindicated. To stop psychomotor agitation and seizures, diprazine, haloperidol, sodium oxybutyrate are prescribed. In severe cases, resort to intubation and mechanical ventilation. It is important to remember that the risk of bleeding is high, so all manipulations must be carried out with extreme caution. When removing the patient from a coma, the next step is to conduct intensive therapy for the underlying disease. Lecture No. 9. Shock Shock is a form of a critical state of the body, manifested by multiple organ dysfunction, developing in a cascade on the basis of a generalized circulation crisis and, as a rule, ending in death without treatment. A shock factor is any effect on the body that exceeds adaptive mechanisms in strength. In shock, the functions of respiration, the cardiovascular system, and kidneys change, the processes of microcirculation of organs and tissues and metabolic processes are disrupted. Etiology and pathogenesis Shock is a disease of a polyetiological nature. Depending on the etiology of occurrence, the types of shock may be different. 1. Traumatic shock: 1) with mechanical injuries - bone fractures, wounds, compression of soft tissues, etc.; 2) with burn injuries (thermal and chemical burns); 3) under the influence of low temperature - cold shock; 4) in case of electrical injuries - electric shock. 2. Hemorrhagic or hypovolemic shock: 1) develops as a result of bleeding, acute blood loss; 2) as a result of an acute violation of the water balance, dehydration of the body occurs. 3. Septic (bacterial-toxic) shock (generalized purulent processes caused by gram-negative or gram-positive microflora). 4. Anaphylactic shock. 5. Cardiogenic shock (myocardial infarction, acute heart failure). Considered in the section emergency conditions in cardiology. In all types of shock, the main mechanism of development is vasodilation, and as a result, the capacity of the vascular bed increases, hypovolemia - the volume of circulating blood (BCC) decreases, since there are various factors: blood loss, redistribution of fluid between the blood and tissues, or a mismatch of the normal blood volume increasing vascular capacity. The resulting discrepancy between the BCC and the capacity of the vascular bed underlies the decrease in cardiac output and microcirculation disorders. The latter leads to serious changes in the body, since it is here that the main function of blood circulation is carried out - the exchange of substances and oxygen between the cell and the blood. There comes a thickening of the blood, an increase in its viscosity and intracapillary microthrombosis. Subsequently, cell functions are disrupted up to their death. In tissues, anaerobic processes begin to predominate over aerobic ones, which leads to the development of metabolic acidosis. Accumulation of metabolic products, mainly lactic acid, increases acidosis. A feature of the pathogenesis of septic shock is a violation of blood circulation under the influence of bacterial toxins, which contributes to the opening of arteriovenous shunts, and blood begins to bypass the capillary bed and rushes from arterioles to venules. Due to a decrease in capillary blood flow and the action of bacterial toxins specifically on the cell, cell nutrition is disrupted, which leads to a decrease in the supply of oxygen to cells. In anaphylactic shock, under the influence of histamine and other biologically active substances, capillaries and veins lose their tone, while the peripheral vascular bed expands, its capacity increases, which leads to pathological redistribution of blood. Blood begins to accumulate in the capillaries and venules, causing a violation of cardiac activity. The BCC formed at the same time does not correspond to the capacity of the vascular bed, and the minute volume of the heart (cardiac output) decreases accordingly. The resulting stagnation of blood in the microcirculatory bed leads to a breakdown in metabolism and oxygen between the cell and blood at the level of the capillary bed. The above processes lead to ischemia of the liver tissue and disruption of its functions, which further exacerbates hypoxia in severe stages of shock development. Violated detoxification, protein-forming, glycogen-forming and other functions of the liver. The disorder of the main, regional blood flow and microcirculation in the renal tissue contributes to the disruption of both the filtration and concentration functions of the kidneys with a decrease in diuresis from oliguria to anuria, which leads to the accumulation of nitrogenous wastes in the patient's body, such as urea, creatinine, and other toxic metabolic products substances. The functions of the adrenal cortex are impaired, the synthesis of corticosteroids (glucocorticoids, mineralocorticoids, androgenic hormones) is reduced, which aggravates the ongoing processes. Circulatory disorders in the lungs explain the violation of external respiration, alveolar gas exchange decreases, blood shunting occurs, microthrombosis is formed, and as a result, the development of respiratory failure, which aggravates tissue hypoxia. Clinic Hemorrhagic shock is a reaction of the body to the resulting blood loss (loss of 25-30% of BCC leads to severe shock). In the occurrence of burn shock, the pain factor and massive plasma loss play a dominant role. Rapidly developing oliguria and anuria. The development of shock and its severity are characterized by the volume and rate of blood loss. Based on the latter, compensated hemorrhagic shock, decompensated reversible shock and decompensated irreversible shock are distinguished. With compensated shock, pallor of the skin, cold sticky sweat, the pulse becomes small and frequent, blood pressure remains within the normal range or is slightly reduced, but slightly, urination decreases. With uncompensated reversible shock, the skin and mucous membranes become cyanotic, the patient becomes lethargic, the pulse is small and frequent, there is a significant decrease in arterial and central venous pressure, oliguria develops, the Algover index is increased, the ECG shows a violation of myocardial oxygen supply. With an irreversible course of shock, consciousness is absent, blood pressure drops to critical numbers and may not be detected, the skin is marble-colored, anuria develops - cessation of urination. The Algover index is high. To assess the severity of hemorrhagic shock, the determination of BCC, the volume of blood loss, is of great importance. The shock severity analysis map and evaluation of the results obtained are shown in Table 4 and Table 5. Table 4 Shock Severity Analysis Map
Table 5 Evaluation of results by total points
The shock index, or Algover index, is the ratio of heart rate to systolic pressure. In shock of the first degree, the Algover index does not exceed 1. In the second degree - no more than 2; with an index of more than 2, the condition is characterized as incompatible with life. Types of shocks Anaphylactic shock is a complex of various allergic reactions of an immediate type, reaching an extreme degree of severity. There are the following forms of anaphylactic shock: 1) cardiovascular form, in which acute circulatory failure develops, manifested by tachycardia, often with heart rhythm disturbances, ventricular and atrial fibrillation, and a decrease in blood pressure; 2) respiratory form, accompanied by acute respiratory failure: shortness of breath, cyanosis, stridor, bubbling breathing, moist rales in the lungs. This is due to a violation of capillary circulation, swelling of the lung tissue, larynx, epiglottis; 3) cerebral form due to hypoxia, impaired microcirculation and cerebral edema. According to the severity of the course, 4 degrees of anaphylactic shock are distinguished. I degree (mild) is characterized by itching of the skin, the appearance of a rash, headache, dizziness, a feeling of flushing to the head. II degree (moderate severity) - Quincke's edema, tachycardia, lowering of arterial pressure, increase of the Algover index join the previously indicated symptoms. Grade III (severe) is manifested by loss of consciousness, acute respiratory and cardiovascular failure (shortness of breath, cyanosis, stridor breathing, small rapid pulse, a sharp decrease in blood pressure, high Algover index). IV degree (extremely severe) is accompanied by loss of consciousness, severe cardiovascular insufficiency: the pulse is not determined, blood pressure is low. Treatment. Treatment is carried out according to the general principles of shock treatment: restoration of hemodynamics, capillary blood flow, the use of vasoconstrictors, normalization of BCC and microcirculation. Specific measures are aimed at inactivating the antigen in the human body (for example, penicillinase or b-lactamase in shock caused by antibiotics) or preventing the effect of the antigen on the body - antihistamines and membrane stabilizers. 1. Intravenous adrenaline infusion until hemodynamic stabilization. You can use dopmin 10-15 mcg / kg / min, and with symptoms of bronchospasm and b-adrenergic agonists: alupent, brikanil drip intravenously. 2. Infusion therapy in a volume of 2500-3000 ml with the inclusion of polyglucin and rheopolyglucin, unless the reaction is caused by these drugs. Sodium bicarbonate 4% 400 ml, glucose solutions to restore bcc and hemodynamics. 3. Membrane stabilizers intravenously: prednisolone up to 600 mg, ascorbic acid 500 mg, troxevasin 5 ml, sodium etamsylate 750 mg, cytochrome C 30 mg (daily doses are indicated). 4. Bronchodilators: eufillin 240-480 mg, noshpa 2 ml, alupent (brikanil) 0,5 mg drip. 5. Antihistamines: diphenhydramine 40 mg (suprastin 60 mg, tavegil 6 ml), cimetidine 200-400 mg intravenously (daily doses are indicated). 6. Protease inhibitors: trasylol 400 thousand U, contrical 100 thousand U. Traumatic shock is a pathological and critical condition of the body that has arisen in response to an injury, in which the functions of vital systems and organs are impaired and inhibited. During trauma shock, torpid and erectile phases are distinguished. By the time of occurrence, shock can be primary (1-2 hours) and secondary (more than 2 hours after injury). Erectile stage or phase of occurrence. Consciousness remains, the patient is pale, restless, euphoric, inadequate, can scream, run somewhere, escape, etc. In this stage, adrenaline is released, due to which pressure and pulse can remain normal for some time. The duration of this phase is from several minutes and hours to several days. But in most cases it is short. The torpid phase replaces the erectile one, when the patient becomes lethargic and adynamic, blood pressure decreases and tachycardia appears. Injury severity estimates are shown in Table 6. Table 6 Assessment of the extent of injury severity
After calculating the points, the resulting number is multiplied by the coefficient. Notes 1. In the presence of injuries that are not specified in the list of the volume and severity of the injury, the number of points is awarded according to the type of injury, according to the severity corresponding to one of the listed ones. 2. In the presence of somatic diseases that reduce the adaptive functions of the body, the found sum of points is multiplied by a coefficient from 1,2 to 2,0. 3. At the age of 50-60 years, the sum of points is multiplied by a factor of 1,2, older - by 1,5. Treatment. The main directions in treatment. 1. Elimination of the action of the traumatic agent. 2. Elimination of hypovolemia. 3. Elimination of hypoxia. Anesthesia is carried out by the introduction of analgesics and drugs, the implementation of blockades. Oxygen therapy, if necessary, tracheal intubation. Compensation for blood loss and BCC (plasma, blood, rheopolyglucin, polyglucin, erythromass). Normalization of metabolism, as metabolic acidosis develops, calcium chloride 10% - 10 ml, sodium chloride 10% - 20 ml, glucose 40% - 100 ml are introduced. Fight against vitamin deficiency (vitamins of group B, vitamin C). Hormone therapy with glucocorticosteroids - prednisolone intravenously once 90 ml, and subsequently 60 ml every 10 hours. Stimulation of vascular tone (mezaton, norepinephrine), but only with a replenished volume of circulating blood. Antihistamines (diphenhydramine, sibazon) are also involved in anti-shock therapy. Hemorrhagic shock is a condition of acute cardiovascular insufficiency that develops after a significant amount of blood loss and leads to a decrease in perfusion of vital organs. Etiology: injuries with damage to large vessels, acute gastric and duodenal ulcers, rupture of an aortic aneurysm, hemorrhagic pancreatitis, rupture of the spleen or liver, rupture of the tube or ectopic pregnancy, the presence of placental lobules in the uterus, etc. According to clinical data and the magnitude of the deficiency of blood volume, the following degrees of severity are distinguished. 1. Not expressed - there are no clinical data, the level of blood pressure is normal. The volume of blood loss is up to 10% (500 ml). 2. Weak - minimal tachycardia, slight decrease in blood pressure, some signs of peripheral vasoconstriction (cold hands and feet). The volume of blood loss is from 15 to 25% (750-1200 ml). 3. Moderate - tachycardia up to 100-120 beats per minute, decrease in pulse pressure, systolic pressure 1-90 mm Hg. Art., anxiety, sweating, pallor, oliguria. The volume of blood loss is from 100 to 25% (35-1250 ml). 4. Severe - tachycardia more than 120 beats per minute, systolic pressure below 60 mm Hg. Art., often not determined by the tonometer, stupor, extreme pallor, cold extremities, anuria. The volume of blood loss is more than 35% (more than 1750 ml). Laboratory in the general analysis of blood, a decrease in the level of hemoglobin, erythrocytes and hematocrit. The ECG shows nonspecific changes in the ST segment and the T wave, which are due to insufficient coronary circulation. Treatment of hemorrhagic shock includes stopping bleeding, the use of infusion therapy to restore BCC, the use of vasoconstrictors or vasodilators, depending on the situation. Infusion therapy involves intravenous administration of fluid and electrolytes in a volume of 4 liters (saline, glucose, albumin, polyglucin). In case of bleeding, transfusion of single-group blood and plasma is indicated in a total volume of at least 4 doses (1 dose is 250 ml). The introduction of hormonal drugs, such as membrane stabilizers (prednisolone 90-120 mg), is shown. Depending on the etiology, specific therapy is carried out. Septic shock is the penetration of an infectious agent from its initial focus into the blood system and its spread throughout the body. The causative agents can be: staphylococcal, streptococcal, pneumococcal, meningococcal and enterococcal bacteria, as well as Escherichia, Salmonella and Pseudomonas aeruginosa, etc. Septic shock is accompanied by a dysfunction of the pulmonary, hepatic and renal systems, a violation of the blood coagulation system, which leads to thrombohemorrhagic syndrome ( Machabeli syndrome), which develops in all cases of sepsis. The course of sepsis is affected by the type of pathogen, this is especially important with modern methods of treatment. Laboratory progressing anemia is noted (due to hemolysis and oppression of hematopoiesis). Leukocytosis up to 12 109 / l, however, in severe cases, as a sharp depression of the hematopoietic organs is formed, leukopenia can also be observed. Clinical symptoms of bacterial shock: chills, high fever, hypotension, dry warm skin - at first, and later - cold and wet, pallor, cyanosis, mental status disorder, vomiting, diarrhea, oliguria. Characterized by neutrophilia with a shift of the leukocyte formula to the left up to myelocytes; ESR increases to 30-60 mm/h or more. The level of blood bilirubin is increased (up to 35-85 µmol/l), which also applies to the content of residual nitrogen in the blood. Blood coagulation and prothrombin index are lowered (up to 50-70%), the content of calcium and chlorides is reduced. The total blood protein is reduced, which is due to albumin, and the level of globulins (alpha-globulins and b-globulins) increases. In the urine, protein, leukocytes, erythrocytes and cylinders. The level of chlorides in the urine is reduced, and urea and uric acid are elevated. Treatment is primarily etiological in nature, therefore, before prescribing antibiotic therapy, it is necessary to determine the pathogen and its sensitivity to antibiotics. Antimicrobial agents should be used at maximum doses. For the treatment of septic shock, it is necessary to use antibiotics that cover the entire spectrum of gram-negative microorganisms. The most rational is the combination of ceftazidime and impinem, which have proven effective against Pseudomonas aeruginosa. Drugs such as clindamycin, metronidazole, ticarcillin, or imipinem are the drugs of choice when a resistant pathogen occurs. If staphylococci are sown from the blood, it is necessary to begin treatment with drugs of the penicillin group. Treatment of hypotension is at the first stage of treatment in the adequacy of the volume of intravascular fluid. Use crystalloid solutions (isotonic sodium chloride solution, Ringer's lactate) or colloids (albumin, dextran, polyvinylpyrrolidone). The advantage of colloids is that when they are introduced, the required filling pressures are reached most quickly and remain so for a long time. If there is no effect, then inotropic support and (or) vasoactive drugs are used. Dopamine is the drug of choice because it is a cardioselective β-agonist. Corticosteroids reduce the overall response to endotoxins, help to reduce fever and give a positive hemodynamic effect. Prednisolone at a dose of 60 to 90 mg per day. Lecture number 10. Acute poisoning Acute poisoning - diseases of chemical etiology, the clinical picture of which develops with a single ingestion of chemicals into the human body in a toxic dose that can cause violations of vital functions and endanger life. According to the cause and place of occurrence, the following types of poisoning are distinguished. 1. Accidental poisoning (accidents) at work - exposure to industrial poisons during an accident or in violation of technical safety rules during the period of work with harmful substances; in everyday life - when there is an erroneous or incorrect use of household chemicals, in case of self-medication with medicinal drugs, their overdose or their erroneous use, with alcohol and drug intoxication, bites of poisonous insects and snakes; medical errors. 2. Deliberate poisoning - suicidal poisoning (true or demonstrative) and criminal poisoning (with the aim of killing or bringing to a helpless state); "police" (when using chemicals and poisons to restore public order) and combat poisoning (when using chemical weapons), including as a result of terrorist acts. According to the number of victims, individual, group and mass acute intoxications are distinguished. The intake of a toxic substance is possible through the mouth (incorporated, oral), through the respiratory tract (inhalation), unprotected skin (percutaneous), by parenteral injection, or when injected into the rectum, vagina, external auditory canal. In the clinical course of acute poisoning, two stages are distinguished: toxicogenic and somatogenic. The essence of the toxic stage is that the active substance in the body has a dose that can cause a specific toxic effect. The somatogenic stage occurs after the elimination or destruction of the toxic substance and manifests itself in the form of "trace" or residual violations of the structural and functional state of organs and systems. General principles for the diagnosis of acute poisoning 1. Clinical diagnosis is based on the following data: 1) inspection of the scene for the discovery of material evidence of poisoning; 2) anamnesis from the words of the victim or others - you can find out what toxic substance the victim took, the time of taking the toxic substance, the dose of the toxic substance taken, the route of penetration of the toxic substance into the body, the circumstances that accompanied the development of poisoning, etc .; 3) studying the clinical picture of the disease to identify specific symptoms of poisoning that are characteristic of a certain toxic substance or group of substances according to the principle of "selective toxicity". 2. Laboratory toxicological diagnostics is aimed at the qualitative or quantitative determination of toxic substances in the biological media of the body (blood, urine, etc.). 3. Pathological diagnostics consists in the detection of specific post-mortem signs of poisoning and is carried out by forensic experts. 1. Methyl alcohol poisoning Methyl alcohol is absorbed by all routes - respiratory, digestive and percutaneous. The lethal dose of methanol when taken orally ranges from 40-250 ml, but taking even 10-20 ml can cause blindness. Poisoning also occurs after taking various alcoholic mixtures containing from 1,5 to 2,5% methanol. After absorption, methanol is distributed throughout all tissues due to its water solubility. The largest amount accumulates in the kidneys and gastrointestinal tract, the smallest in the brain, muscles and adipose tissue. Pathological anatomical lesions include cerebral edema and damage to the initially inflammatory, and later dystrophic, nature of retinal ganglion cells. In some cases, in addition to damage to the nervous system, degenerative lesions of the liver, kidneys, lungs and heart muscle are found. clinical picture In the toxic effect of methanol, two-phase action can be distinguished. In the initial period (phase I), methanol acts on the body as a whole molecule and has a predominantly narcotic effect, but weaker than that of ethyl alcohol. Subsequently (phase II), the harmful effect of oxidation products is manifested. In the clinical picture, periods are distinguished: intoxication, latent, or relative well-being (lasting from several hours to 1-2 days), the main manifestations of intoxication and, with a favorable outcome, reverse development. According to the severity, mild, moderate (or ophthalmic) and severe (or generalized) are distinguished. With mild poisoning, rapid fatigue, headache, nausea, and a state of slight intoxication are noted, which occur after a latent period of 30 minutes to several hours. The simultaneous absorption of ethyl and methyl alcohol increases the latent period and reduces the severity of poisoning. In case of moderate poisoning, after a short latent period, the patient develops a headache, dizziness, acute pain in the abdomen (mainly in the epigastric region), vomiting, restlessness, delirium, convulsions. Ultimately, a deep coma sets in. The patient has hypothermia, in some cases, cyanosis, when respiratory failure of a central nature develops, followed by cardiovascular failure. At this stage, the pupils dilate, and the study of the fundus indicates the presence of retinal edema. Typical visual disturbances, such as reduced visual acuity, narrowing of the field, dilated pupils, loss of reflex to light, pain in the eyeball occur on the 2nd-6th day. Examination of the fundus reveals signs of optic nerve atrophy. The most severe complication is complete blindness. Treatment Treatment includes the following points. 1. Stopping the entry of poison into the body. Cessation of the use of poison, removal from the contaminated atmosphere, removal of poison from the skin. 2. Removal of non-absorbed poison (from the gastrointestinal tract): 1) probe gastric lavage; 2) the use of adsorbents or saline laxatives; 3) cleansing or siphon enema. 3. Removal of absorbed poison: 1) forcing diuresis; 2) methods of extracorporeal detoxification (hemosorption, hemodialysis, peritoneal dialysis, blood replacement operation). 4. Antidote therapy, i.e. neutralization of the poison due to physical or chemical neutralization, as well as competition with the poison for application points. 6. Maintaining the vital functions of the body. 7. Normalization of the water-electrolyte, acid-base state of the body. 8. Treatment of long-term consequences and complications. Gastric lavage with water or 2% sodium bicarbonate solution, followed by ingestion of 30 g of magnesium sulfate (sodium) in 100 ml of water. Subcutaneously 2 ml cordiamine, 1 ml 20% caffeine solution. In coma, intravenously, a 5% solution of ethyl alcohol in a 5% glucose solution at the rate of 1,5-2 g of alcohol per 1 kg of the patient's weight per day. Intravenously 400 ml of polyglucin (rheopolyglucin), 50-100 mg of prednisolone, glucosone-vocaine mixture (400 ml of 5% glucose solution with 25 ml of 2% novocaine solution), 80-120 mg of furosemide (lasix), 40 ml of 40% - glucose solution with 8 IU of insulin. 2. Ethyl alcohol poisoning Clinic When taking toxic doses - agitation, ataxia, stupor, coma with inhibition of reflexes, the smell of alcohol from the mouth, flushing of the face, conjunctivitis, "play" of the pupils, vomiting, involuntary urination, tachycardia, "hoarse" breathing, collapse, vomiting with possible aspiration of emetic wt. Treatment Gastric lavage through a thick probe, followed by the introduction of a saline laxative, siphon enema. Subcutaneously 1 ml of a 0,1% solution of atropine, 2 ml of cordiamine, 1 ml of a 20% solution of caffeine, with collapse - intramuscularly 1 ml of a 1% solution of mezaton. In the absence of pharyngeal reflexes - tracheal intubation and mechanical ventilation. Forcing diuresis with the simultaneous administration of a 4% sodium bicarbonate solution in a volume calculated according to the Astrup formula, hypertonic (10- and 20%) glucose solutions with insulin, B and C vitamins, cocarboxylase, nicotinic acid. With respiratory depression - intravenously slowly 3-5 ml of a 1,5% solution of etimizole, 10 ml of a 2,4% solution of aminophylline, 1 ml of a 5% solution of ephedrine, oxygen inhalation. With aspiration - emergency sanitation bronchoscopy. Parenteral antibiotics. 3. Ethylene glycol (antifreeze) poisoning The mean lethal dose is approximately 100 ml. It is rapidly absorbed in the digestive tract and distributed to all tissues, creating a maximum concentration in the brain. The main toxic effect is observed in the renal parenchyma, where necrosis of the tubular epithelium develops, interstitial edema, and foci of hemorrhagic necrosis in the cortical layer. Edema is found in the brain. Clinic In the clinic of intoxication, 3 periods are distinguished: 1) initial - lasting up to 12 hours, symptoms of CNS damage by the type of alcohol intoxication predominate; 2) neurotoxic - when the symptoms of CNS damage progress and respiratory and cardiovascular system disorders join; 3) nephrotoxic - on the 2nd-5th day, kidney damage predominates in the clinical picture of intoxication. The duration and severity of periods of intoxication depends on the severity of poisoning. In mild cases, after a period of intoxication (10-15 hours), a slight malaise, headache, and nausea develop. Consciousness remains clear, kidney damage is not observed. In case of poisoning of moderate severity, against the background of symptoms of intoxication, vomiting, diarrhea, pain in the epigastric region, and headache occur. On the 3-5th day, symptoms of kidney damage appear. In severe poisoning, loss of consciousness quickly occurs, stiff neck, clonic-tonic convulsions, fever, noisy deep breathing, collapse, and pulmonary edema occur. If the patient does not die on the first day, then from the 2-3rd day symptoms of renal failure develop: thirst appears, back pain, the amount of urine decreases, up to anuria. The liver is enlarged, painful. Death comes from uremia. Treatment Gastric lavage with water or 2% sodium bicarbonate solution, followed by the introduction of 30 g of magnesium sulfate in 200 ml of water. Inside 200 ml of a 30% solution of ethyl alcohol. 3-5 g of sodium bicarbonate in 100 ml of water. Plentiful drink. Intramuscularly 1-2 ml of cordiamine, 1 ml of a 20% caffeine solution. Intravenously 400 ml of 5% glucose solution with 5 ml of 5% ascorbic acid solution and 8 units of insulin, glucosone-vocaine mixture, 400 ml of polyglucin, 400 ml of hemodez, 80-120 g of furosemide, 60-100 mg of prednisolone. 4. Dichloroethane poisoning The main routes of entry are the digestive tract, respiratory tract, and skin. The lethal dose of DCE for humans when administered orally is 10-20 ml. The toxic effect of DCE is due to the narcotic effect on the central nervous system, damage to the liver, kidneys, gastrointestinal tract, and a pronounced effect on the cardiovascular system. Clinic In the clinical picture, the leading symptoms are: toxic encephalopathy, acute toxic gastritis and gastroenteritis, impaired external respiration, toxic hepatitis, impaired renal function. In the first hours after administration, dizziness, ataxia, psychomotor agitation, clonic-tonic convulsions, depression of consciousness, up to a coma, appear. One of the early signs of poisoning are gastrointestinal disorders in the form of nausea, repeated vomiting with an admixture of bile. Violation of the function of external respiration often occurs according to the obstructive-aspiration type and is associated with increased salivation, bronchorrhea, and aspiration. Inhibition of the function of the respiratory center, the development of hypertonicity of the respiratory muscles and rigidity of the chest are noted. A severe complication is exotoxic shock, which is manifested by cooling and cyanosis of the skin, cold sweat, shortness of breath, tachycardia and hypovolemia. Decreased excretory function of the kidneys. According to the severity of DCE poisoning are divided into the following. 1. Mild poisoning, characterized by the development of dyspeptic disorders, mild neurological symptoms and mild hepatopathy. 2. Moderate poisoning. Occur with symptoms of toxic gastritis, gastroenteritis, toxic encephalopathy, moderate hepatopathy, mild nephropathy. 3. Severe poisoning occurs with severe manifestations of toxic encephalopathy, severe hepatopathy, toxic nephropathy, gastroenteritis. In case of vapor poisoning, headache, drowsiness, a sweetish taste in the mouth, the smell of poison from the mouth, irritation of the mucous membranes, loss of consciousness, acute hepatic-renal failure with yellowness of the sclera and skin, enlarged liver, anuria occur. When ingested - repeated vomiting, abdominal pain, loose stools with the smell of poison, hyperemia of the sclera, psychomotor agitation, followed by depression and coma, collapse, toxic shock, hemorrhagic syndrome, acute hepatic-renal failure. When the poison gets on the skin, dermatitis develops. Treatment In case of vapor poisoning - the removal of the victim from the affected area, with respiratory depression of the ventilator. If the poison gets inside - gastric lavage through a thick probe, followed by the introduction of 3-4 tablespoons of powdered activated carbon in 200 ml of water and 150-200 ml of vaseline oil, siphon enema. Intravenously 20-40 ml of a 30% solution of sodium thiosulfate, intramuscularly 5 ml of a 5% solution of unitiol. intravenously 400 ml of polyglucin, 400 ml of hemodez, 400 ml of 5% glucose solution, 80-120 mg of furosemide (lasix), 6-8 ml of 5% solution of ascorbic acid. Intramuscularly 2 ml of cordiamine, with collapse - 1 ml of a 1% solution of mezaton, 1 ml of a 20% solution of caffeine, 100-150 mg of prednisolone. With an increase in hepatic-renal failure, specific therapy is carried out. Intravenously bolus 400 ml of polyglucin, 400 ml of gemodez, 400 ml of 5% glucose, 80-120 mg of furosemide, 6-8 ml of 5% ascorbic acid, 100-150 mg of prednisolone, with collapse 1 ml of 1% mezaton . Intramuscularly 2-4 ml of 6% thiamine bromide and 2-4 ml of 5% pyridoxine. With psychomotor agitation, 1 ml of a 3% solution of fenozepam. Oxygen inhalation, with respiratory depression - IVL through a breathing tube. During the first day, the most effective and sparing method is peritoneal dialysis. The composition of the standard dialysis solution includes: potassium chloride 0,3 g, sodium chloride 8,3 g, magnesium chloride 0,1 g, calcium chloride 0,3 g, glucose 6 g per 1 liter of water. At the same time, up to 2 liters of electrolyte solution with the addition of 500 thousand units of penicillin and 1000 units of heparin are injected into the patient's abdominal cavity. In the somatogenic phase of poisoning, the main therapy is aimed at treating the developed complications: pneumonia, hepatopathy, etc. 5. Poisoning with poisonous mushrooms (fly agaric, false mushrooms, morels, pale grebe) Clinic After a latent period lasting from 1-2 to 36 hours, cramping abdominal pain, salivation, nausea, indomitable vomiting, diarrhea, dehydration, collapse, delirium, hallucinations, convulsions appear. On the 2-3rd day - the phenomena of renal and hepatic insufficiency with anuria, azotemia, jaundice. In severe poisoning with lines and morels, hemolysis is possible. Treatment Gastric lavage through a thick tube, followed by the introduction of 3-4 tablespoons of powdered activated carbon in 200 ml of water and 30 g of magnesium sulfate (sodium) in 100 ml of water, siphon enema. Subcutaneously 1 ml of a 0,1% solution of atropine, 2 ml of cordiamine. In morel poisoning, atropine is not used as an antidote. Intravenously 400 ml of polyglucin, 400 ml of hemodez, 400 ml of 5% glucose solution with 4-6 ml of 5% ascorbic acid solution, 80-120 mg of furosemide (lasix). Intramuscularly 1-2 ml of a 6% solution of thiamine bromide and 2 ml of a 5% solution of pyridoxine hydrochloride (do not inject in one syringe). Relief of pain syndrome intramuscularly with the introduction of 1 ml of a 0,2% solution of platifillin, 2 ml of a 2% solution of papaverine. With convulsions, psychomotor agitation - intramuscularly 1-2 ml of a 3% solution of fenozepam or a lytic mixture (1-2 ml of a 2,5% solution of chlorpromazine, 1-2 ml of a 1% solution of dimedrol, 5-10 ml 25 % solution of magnesium sulfate) under the control of blood pressure. Forcing diuresis. In severe cases - hemosorption, early hemodialysis. In case of severe poisoning with strings and morels, in the case of severe hemolysis and the absence of the possibility of hemodialysis, blood replacement surgery is performed. Correction of acidosis by intravenous administration of a 4% solution of sodium bicarbonate in a volume determined according to the Astrup formula. Antibiotics - benzylpenicillin up to 10 million units per day. Symptomatic therapy. With increasing hepatic and renal insufficiency - infusion therapy with a 5-10% glucose solution with insulin, vitamins of group B and C, 20-30 thousand IU of heparin, aminophylline. 6. Snake venom poisoning Clinic Pain and rapidly spreading swelling at the site of the bite, drowsiness, respiratory depression, collapse, intravascular hemolysis with hemoglobinuria, severe subcutaneous hemorrhages, sometimes convulsions. Possibly kidney failure. With a cobra bite, local changes are less pronounced, bulbar disorders (speech and swallowing disorders, ptosis, paralysis of the motor muscles) and respiratory depression predominate. Shows complete rest in a horizontal position. Squeezing out the first drops of blood from the wound. Immobilization of the injured limb. In place of the bite - cold. Plentiful drink. Treatment Suction of blood and lymph from the wound (no later than 30-60 minutes after the bite), formed at the site of the bite, using a blood-sucking jar. Washing the wound with 1% potassium permanganate solution. Introduction to the wound 0,3-0,5 ml of a 0,1% solution of adrenaline. If possible, urgent introduction of a specific mono- or polyvalent anti-snake serum after a preliminary intravenous injection of 100-150 ml of hydrocortisone or 50-100 ml of prednisolone. In case of cobra bites - intravenous Anticobra serum at a dose of 300 ml in combination with 1 ml of a 0,05% solution of prozerin and repeated administration every 30 minutes with 1 ml of a 0,1% solution of atropine. Prophylactically - anti-tetanus serum according to Bezredka, intravenous polarizing mixture, 10-15 thousand units of heparin, 1-2 ml of 1% diphenhydramine solution, 10 ml of 10% calcium chloride (gluconate) solution, 5-10 ml of 5% - ascorbic acid solution, 50-100 mg of prednisolone or 100-150 mg of hydrocortisone, 400 ml of gemodez, 400 ml of polyglucin, 40-80 mg of lasix. Pain relief by intravenous administration of 1 ml of a 2% solution of promedol. In acute respiratory failure - mechanical ventilation through a breathing tube, oxygen inhalation. In severe toxicosis - hemosorption, with the progression of hepatic-renal failure - hemodialysis in combination with hemosorption. Symptomatic therapy. 7. Poisoning with concentrated acids (nitric, acetic, sulfuric) Clinic Inhalation of vapors causes irritation of the eyes and upper respiratory tract (lacrimation, runny nose, cough, shortness of breath). Reflex respiratory arrest is possible. After a latent period (from 2 to 24 hours), toxic pneumonia or toxic pulmonary edema is formed. At hit in eyes, on skin - chemical burns. When ingested - a chemical burn of the oral cavity, pharynx, esophagus, stomach, possible swelling of the larynx with respiratory failure. Repeated vomiting with blood, peritoneal irritation, and occasionally perforation of the esophagus or stomach. Collapse, shock, hemorrhagic syndrome. Possible intravascular hemolysis, hemoglobinuric nephrosis with acute renal (renal-hepatic) insufficiency. Tubeless gastric lavage and artificial vomiting are dangerous due to the possibility of re-burning the esophagus and acid aspiration. Do not inject saline laxative and alkaline solutions. Treatment Gastric lavage through a thick tube with cold water after a preliminary intravenous or intramuscular injection of 1-2 ml of a 2% solution of promedol. Inside pieces of ice, Almagel A 15-20 ml every hour. Intravenously 800 ml of polyglucin, 400 ml of hemodez, glucosone-vocaine mixture (400 ml of 5% glucose solution with 25 ml of 2% novocaine solution), 50-150 mg of prednisolone or 150-250 mg of hydrocortisone, 10 thousand units of heparin, 80-120 mg furosemide (Lasix). Relief of the pain syndrome is achieved intravenously with the introduction of 1-2 ml of a 0,005% solution of fentanyl and 2-4 ml of a 0,25% solution of droperidol, with persistent pain in the abdomen - intramuscularly 1-2 ml of a 0,2% solution of platyfillin, 2 ml of 2% papaverine solution. Inhalation of oxygen with a defoamer. With increasing laryngeal edema - intravenously 200-400 mg of prednisolone, 1-2 ml of a 1% solution of diphenhydramine, 10-20 ml of a 2,4% solution of aminophylline, 1-2 ml of a 5% solution of ephedrine. In the absence of effect - tracheostomy, oxygen inhalation, according to indications - mechanical ventilation. 8. Poisoning with arsenic and its compounds Clinic There is a metallic taste in the mouth, abdominal pain, vomiting, loose stools, severe dehydration, convulsions, tachycardia, lowering blood pressure, coma, acute renal failure. Arsenic hydrogen poisoning develops intravascular hemolysis, hemoglobinuria. Treatment Gastric lavage through a thick tube (2-3 times a day) with the introduction at the beginning and at the end of washing 50 ml of a 5% solution of unithiol, repeated siphon enemas with the addition of unithiol. Continuation of antidote therapy intravenously or intramuscularly with the introduction of a 5% solution of unitiol (up to 300 ml per day), intravenous drip of 20 ml of 10% calcium tetacine (ED1A) in 400 ml of a 5% glucose solution. In case of poisoning with arsenic hydrogen - intramuscularly, 1-2 ml of a 40% solution of mecaptide (up to 6-8 ml per day). Forcing diuresis with the simultaneous administration of hypertonic (10-20% glucose solution) polyionic solutions, 4% sodium bicarbonate solution in a volume calculated according to the Astrup formula, glucosone-vocaine mixture, aminophylline, B and C vitamins, cytochrome C. In severe poisoning - early hemodialysis. In the process of performing hemodialysis, a 5% unithiol solution is injected intravenously (30-40 ml / h for severe poisoning, 20-30 ml / h for moderate poisoning). In acute hemolysis and the impossibility of hemodialysis, a blood replacement operation is performed. 9. Lye poisoning Clinic When ingested, a chemical burn of the mucous membrane of the oral cavity, esophagus, and stomach develops. Pain along the esophagus and in the abdomen, vomiting with an admixture of blood, esophageal-gastric bleeding. Possible perforation of the esophagus, stomach with the development of mediastinitis, peritonitis. With a burn of the larynx - hoarseness of voice, aphonia, difficulty (stridor) breathing. In severe cases - burn shock, oliguria. Contact with skin causes chemical burns. Treatment Treat as in acid poisoning. 10. Atropine poisoning Clinic Dry mouth, hoarseness, dry, hyperemic skin, dilated pupils, shortness of breath, palpitations, tachycardia, thirst, nausea, difficulty urinating. In severe poisoning - psychomotor agitation, delirium, hallucinations, convulsions, heart rhythm disturbances, coma, collapse are possible. Treatment If necessary - gastric lavage through a thick probe, richly lubricated with vaseline oil, the introduction of 3-4 tbsp. l. powdered activated carbon in 200 ml of water and 30 mg of magnesium sulfate in 100 ml of water. intravenously 2-4 ml of 0,05% proserin solution, 400-800 ml of 5% glucose solution, 40-80 mg of furosemide (lasix). Plentiful drink. Relief of psychomotor agitation and convulsions intramuscularly with the introduction of 1-2 ml of a 3% solution of fenozepam or a lytic mixture (2 ml of a 2,5% solution of chlorpromazine, 2 ml of a 1% solution of diphenhydramine and 10 ml of a 25% solution of magnesium sulfate ) or 1-2 g of chloral hydrate in an enema with 1-2 g of starch per 25-50 ml of water, intravenously 10-15 ml of a 20% sodium hydroxybutyrate solution, 2-4 ml of a 0,5% solution of seduxen. With severe tachycardia, extrasystole - intravenously anaprilin (1-2 ml of a 0,25% solution) or an anaprilin tablet (40 mg) under the tongue. With collapse - intravenously 1 ml of a 1% solution of mezaton in 10 ml of a 0,9% solution of sodium chloride. With a sharp hyperthermia - intramuscularly 2 ml of a 50% solution of analgin, ice packs on large vessels and the head, wet wraps. 11. Hemp poisoning (hashish, marijuana, marijuana, plan) Clinic There is euphoria, psychomotor agitation, vivid visual hallucinations, dilated pupils, tinnitus. Subsequently, weakness, lethargy, depression of mood, drowsiness, bradycardia, hypothermia. Treatment In case of oral poisoning - gastric lavage through a thick probe, followed by the introduction of 3-4 tablespoons of powdered activated carbon in 200 ml of water. Intravenously 400-800 ml of a 5% glucose solution with 5-10 ml of a 5% solution of ascorbic acid and 8-16 units of insulin, 40-80 mg of furosemide (lasix), intramuscularly 2 ml of a 6% solution of thiamine bromide. With a sharp excitation - intramuscularly 3-5 ml of a 2,5% solution of chlorpromazine or a lytic mixture (2 ml of a 2,5% solution of chlorpromazine, 1-2 ml of a 1% solution of diphenhydramine and 5-10 ml of 25% - a solution of magnesium sulfate) under the control of blood pressure. oxygen inhalation. Forcing diuresis. In severe cases resort to hemosorption. 12. Cocaine and dicaine poisoning Clinic Clinically manifested by general agitation, headache, flushing of the face, dilated pupils, tachycardia, increased respiration, increased blood pressure, hallucinations. In severe cases - convulsions, coma, respiratory paralysis, collapse. Treatment Repeated gastric lavage through a thick probe with a 0,1% solution of potassium permanganate, followed by the introduction of 3-4 tbsp. l. powdered activated carbon in 200 ml of water and 30 g of magnesium sulfate in 100 ml of water. Intravenously 400 ml of gemodez, 400 ml of 5% glucose solution with 5-10 ml of 5% ascorbic acid solution, 40-80 mg of furosemide (lasix). When excited - fixing the victim, intramuscularly 1-2 ml of a 3% solution of fenozepam or a lytic mixture (1-2 ml of a 2,5% solution of chlorpromazine, 2 ml of a 1% solution of diphenhydramine and 5-10 ml of 25% - a solution of magnesium sulfate) under the control of blood pressure. With convulsions, 1-2 g of chloral hydrate is administered in an enema with 1-2 g of starch in 25-50 ml of water, slowly intravenously 15-20 ml of a 20% sodium oxybutyrate solution, if there is no effect, slowly intramuscularly up to 20 ml of 2,5% solution of sodium thiopental or hexenal. With the development of a coma - an ice pack on the head, intravenously 40 ml of a 40% glucose solution with 4-6 ml of a 5% solution of ascorbic acid and 8 units of insulin, intravenously slowly or intramuscularly 2-4 ml of a 6% solution of thiamine bromide and 2-4 ml of a 5% solution of pyridoxine hydrochloride, 80-120 mg of furosemide. With severe respiratory depression, mechanical ventilation is carried out, intravenously slowly 2 ml of cordiamine, oxygen inhalation. 13. Poisoning with narcotic analgesics (morphine, omnopon, droperidol) Clinic Drowsiness or unconsciousness, pupillary constriction, muscle hypertonicity (sometimes convulsions), respiratory depression, bradycardia, collapse. Respiratory paralysis is possible with the patient's consciousness preserved. Treatment Gastric lavage through a thick probe (while maintaining consciousness), followed by the introduction of 3-4 tablespoons of powdered activated carbon and 30 g of sodium sulfate, siphon enema. Intravenously 400 ml of gemodez, 400 ml of polyglucin, 400 ml of 5% glucose solution, 60-80 mg of furosemide (lasix). Subcutaneously 1-2 ml of a 0,1% solution of atropine, 1-2 ml of cordiamine, 1 ml of a 20% solution of caffeine. With the development of a coma, an ice pack on the head, intravenously 40 ml of a 40% glucose solution with 5-10 ml of a 5% solution of ascorbic acid and 8 units of insulin, intravenously slowly or intramuscularly 2-4 ml of a 6% solution of thiamine bromide and 2-4 ml of a 5% solution of pyridoxine hydrochloride, 80-120 mg of furosemide (lasix). If necessary - bladder catheterization and urine extraction. With respiratory depression - IVL, oxygen inhalation. Hemosorption (2-3 times a day until consciousness is restored). Lecture No. 11. Pain and analgesics 1. Pain Pain is an unpleasant sensory and emotional state caused by real or potential pathological effects on tissues. In the CNS, pain is conducted along two main pathways. Specific path - the posterior horns of the spinal cord, specific nuclei of the thalamus, the cortex of the posterior central gyrus. This pathway is low-neuronal, fast, conducts threshold, emotionally uncolored, precisely localized pain (epicritic pain). Non-specific way - the posterior horns of the spinal cord, non-specific nuclei of the thalamus, the cortex of the frontal and parietal lobes diffusely. Conducts subthreshold, emotionally colored, poorly localized pain. It is slow, multineuronal, as it forms numerous collaterals to the medulla oblongata, the reticular formation, the limbic system, and the hippocampus. Subthreshold pain impulses undergo summation in the thalamus. Impulses conducted along the nonspecific pain pathway excite the emotional centers of the limbic system, the autonomic centers of the hypothalamus, and the medulla oblongata. Therefore, pain is accompanied by fear, painful experiences, increased respiration, pulse, rise in blood pressure, pupil dilation, dyspeptic disorders. The action of the nociceptive pain system is counteracted by the antinoceceptive system, the main neurons of which are localized in the periaqueductal gray matter (the aqueduct of Sylvius connects the III and IV ventricles). Their axons form descending pathways to the medulla oblongata and spinal cord and ascending pathways to the reticular formation, thalamus, hypothalamus, limbic system, basal ganglia, and cortex. The mediators of these neurons are pentapeptides: methenkephalin and leuenkephalin, which have methionine and leucine as terminal amino acids, respectively. Enkephalins excite opiate receptors. In enkephalinergic synapses, opiate receptors are located on the postsynaptic membrane, but the same membrane is presynaptic for other synapses. Opiate receptors are associated with adenylate cyclase and cause its inhibition by disrupting cAMP synthesis in neurons. As a result, calcium entry and release of mediators, including pain mediators - peptides decreases: substance P, cholecystokinin, somatostatin, glutamic acid. Opiate receptors are excited not only by mediators - enkephalins, but also by other components of the antinoceceptive system - brain hormones (endorphins). Peptide agonists of opiate receptors are formed during proteolysis of the peptide substances of the brain: proopiocortin, proenkephalins A and B. All these peptides are formed in the hypothalamus. Opiate receptors excite receptors in all brain structures involved in the conduction and perception of pain, the formation of emotionally colored reactions to pain. At the same time, the release of pain mediators decreases and all reactions accompanying pain are weakened. 2. Analgesic drugs An analgesic (acetylsalicylic acid, paracetamol, morphine) is a drug that reduces pain of various origins. Drugs that reduce pain provoked only by a certain causative factor, or eliminate a specific pain syndrome, for example, antacids, ergotamine (migraine), carbamazepine (neuralgia), nitroglycerin (angina pectoris), do not belong to classical analgesics. Corticosteroids suppress the inflammatory response and the resulting pain, but despite their widespread use for these purposes, they also do not represent classical analgesics. Analgesics are classified into narcotic, acting on CNS structures and causing drowsiness, such as opioids, and non-narcotic, acting mainly on peripheral structures, such as paracetamol, acetylsalicylic acid. Additional drugs that enhance the effect of analgesics The drugs of this group are not analgesics themselves, but are used in combination with analgesics for pain, as they can change the attitude towards pain, its perception and level anxiety, fear, depression (tricyclic antidepressants can even cause a decrease in the need for morphine in a patient in terminal state). Such drugs can be psychotropic drugs, as well as those that affect the mechanisms of pain, for example, eliminating spasm of smooth and striated muscles. Narcotic analgesics are herbal and synthetic drugs that selectively reduce the perception of pain, increase pain tolerance by reducing the emotional coloring of pain and its vegetative accompaniment, cause euphoria and drug dependence. Narcotic analgesics reduce the conduction and perception of pain only within the boundaries of the central nervous system, suppressing mainly the nonspecific pathway. Means of this group excite opiate receptors, create an action similar to the effects of peptides of the anti-noreceptive system. Therefore, the main mechanisms of anesthesia are the following: a disorder in the conduction of a pain impulse from the axon of neuron I, whose body is located in the spinal ganglion, to neuron II, located in the gelatinous substance of the posterior horns of the spinal cord. Suppression of summation of subthreshold impulses in the thalamus. Decreased participation in the pain reaction of the medulla oblongata, hypothalamus, limbic system (non-accentuated attitude to pain). Classification of narcotic analgesics and their antagonists The classification is as follows. 1. Piperidine-phenanthrene derivatives: 1) morphine; 2) codeine (methylmorphine, 5-7 times weaker than morphine as an analgesic); 3) ethylmorphine (dionine, equal in strength to morphine). 2. Phenylpiperidine derivatives: 1) promedol (3-4 times weaker than morphine); 2) fentanyl (100-400 times stronger than morphine). 3. Derivatives of diphenylmethane: 1) pyritramide (dipidolor) - equal to morphine; 2) tramadol (tramal) - somewhat inferior to morphine. 4. Agonists-antagonists: 1) opiate receptor agonists and opiate receptor antagonists - buprenorphine (norphine) (25-30 times stronger than morphine); 2) opiate receptor agonists and opiate receptor antagonists - pentazocine (lexir) (2-3 times weaker than morphine) and butorphanol (moradol) (equal to morphine). Agonists-antagonists are much less likely and weaker than full agonists to cause euphoria and drug dependence. Narorphine - on its own (for example, with barbiturate poisoning) and with mild morphine poisoning, it has an analgesic effect, causes miosis, bradycardia, and exacerbates respiratory center depression. In severe poisoning with morphine and other agonists, it displaces them from the opiate receptors of the respiratory center and restores breathing. Causes dysphoria, irritability, depression, impaired focusing of the gaze. Complete opioid receptor antagonists Naloxone has no independent action, it is effective as an antidote for poisoning with narcotic analgesics. Narcotic analgesics should be used only for acute pain for a short time. Most often used for injuries, burns, myocardial infarction, peritonitis (after clarifying the diagnosis and deciding on the operation). Narcotic analgesics are part of lytic mixtures for potentiation of anesthesia. The drugs of this group are used for postoperative pain in combination with M-anticholinergics and myotropic antispasmodics. They are prescribed to stop hepatic (pentazocine) and renal (promedol) colic. Chronic pain is a contraindication for prescribing drugs, with the exception of advanced forms of a malignant tumor (dipidolor, tramadol, agonists-antagonists). Narcotic analgesics are combined with psychotropic drugs for special types of anesthesia. Neuroleptanalgesia is pain relief with a combination of fentanyl (strong, lasts 30-40 minutes) and droperidol (a mild antipsychotic). Droperidol has a mild sedative effect, stops emotional reactions and reduces the tone of skeletal muscles. Also important effects of droperidol are antiemetic and antishock. Doses of droperidol - 1: 50. Combined drug - thalamonal. Neuroleptanalgesia is used in low-traumatic operations, in the field of neurosurgery and in cardiology for myocardial infarction, etc. Atalgesia or tranquilizer analgesia - fentanyl in combination with a strong tranquilizer such as sibazon, phenazepam. The main disadvantage is the strong respiratory depression of fentanyl and the preservation of consciousness. Lecture No. 12. Anesthesia. Types and stages of anesthesia General anesthesia, or anesthesia, is a state of the body that is characterized by a temporary shutdown of a person’s consciousness, his pain sensitivity and reflexes, as well as relaxation of the muscles of the skeletal muscles, caused by the action of narcotic analgesics on the central nervous system. Depending on the routes of administration of narcotic substances into the body, inhalation and non-inhalation anesthesia are distinguished. 1. Theories of anesthesia Currently, there are no theories of anesthesia that would clearly define the narcotic mechanism of action of anesthetics. Among the available theories of anesthesia, the most significant are the following. Drugs can cause specific changes in all organs and systems. During the period when the body is saturated with a narcotic analgesic, there is a certain staging in the change in consciousness, respiration and blood circulation of the patient. Therefore, there are stages that characterize the depth of anesthesia. These stages manifest themselves especially clearly during ether anesthesia. There are 4 stages: 1) analgesia; 2) excitement; 3) surgical stage, subdivided into 4 levels; 4) stage of awakening. Stage of analgesia The patient is conscious, but some lethargy is noted, he is dozing, answers questions in monosyllables. Superficial and pain sensitivity are absent, but as for tactile and thermal sensitivity, they are preserved. In this stage, short-term surgical interventions are performed, such as opening phlegmon, abscesses, diagnostic studies, etc. The stage is short-term, lasting 3-4 minutes. Excitation stage In this stage, the centers of the cerebral cortex are inhibited, and the subcortical centers at this time are in a state of excitation. At the same time, the patient's consciousness is completely absent, pronounced motor and speech excitation is noted. Patients begin to scream, make attempts to get up from the operating table. Hyperemia of the skin is noted, the pulse becomes frequent, systolic blood pressure rises. The pupil of the eye becomes wide, but the reaction to light persists, lacrimation is noted. Often there is a cough, increased bronchial secretion, sometimes vomiting. Surgical intervention against the background of excitation cannot be performed. During this period, you should continue to saturate the body with a narcotic to enhance anesthesia. The duration of the stage depends on the general condition of the patient and the experience of the anesthesiologist. Typically, the duration of excitation is 7-15 minutes. Surgical stage With the onset of this stage of anesthesia, the patient calms down, breathing becomes calm and even, heart rate and blood pressure approach normal. During this period, surgical interventions are possible. Depending on the depth of anesthesia, 4 levels and stage III of anesthesia are distinguished. First level: the patient is calm, the number of respiratory movements, the number of heartbeats and blood pressure are approaching the initial values. The pupil gradually begins to narrow, its reaction to light is preserved. There is a smooth movement of the eyeballs, an eccentric arrangement. The corneal and pharyngeal-laryngeal reflexes were preserved. Muscle tone is preserved, therefore abdominal operations at this level are not performed. Second level: the movement of the eyeballs is stopped, they are fixed in a central position. The pupils dilate, and their reaction to light weakens. The activity of the corneal and pharyngeal-laryngeal reflexes begins to weaken with a gradual disappearance towards the end of the second level. Respiratory movements are calm and even. Values of arterial pressure and pulse acquire normal values. Muscle tone is reduced, which allows for abdominal operations. Anesthesia, as a rule, is carried out in the period of the first and second levels. The third level is characterized as deep anesthesia. At the same time, the pupils of the eyes are dilated with a reaction to a strong light stimulus. As for the corneal reflex, it is absent. Complete relaxation of the skeletal muscles develops, including the intercostal muscles. Due to the latter, respiratory movements become superficial or diaphragmatic. The lower jaw sags, as its muscles relax, the root of the tongue sinks and closes the entrance to the larynx. All of the above leads to respiratory arrest. In order to prevent this complication, the lower jaw is brought forward and held in this position. At this level, tachycardia develops, and the pulse becomes small filling and tension. The level of arterial pressure decreases. Carrying out anesthesia at this level is dangerous for the life of the patient. fourth level; the maximum expansion of the pupil with the absence of its reaction to light, the cornea is dull and dry. Given that paralysis of the intercostal muscles develops, breathing becomes superficial and is carried out by movements of the diaphragm. Tachycardia is characteristic, while the pulse becomes threadlike, frequent and difficult to determine in the periphery, blood pressure is sharply reduced or not detected at all. Anesthesia at the fourth level is life-threatening for the patient, as respiratory and circulatory arrest may occur. Awakening stage As soon as the introduction of narcotic drugs stops, their concentration in the blood decreases, and the patient goes through all the stages of anesthesia in reverse order, awakening occurs. 2. Preparing the patient for anesthesia The anesthesiologist takes a direct and often the main role in preparing the patient for anesthesia and surgery. An obligatory moment is the examination of the patient before the operation, but at the same time, not only the underlying disease, for which surgery is to be performed, but also the presence of concomitant diseases, which the anesthesiologist asks in detail, is important. It is necessary to know how the patient was treated for these diseases, the effect of treatment, the duration of treatment, the presence of allergic reactions, the time of the last exacerbation. If the patient undergoes a surgical intervention in a planned manner, then, if necessary, correction of existing concomitant diseases is carried out. Sanitation of the oral cavity is important in the presence of loose and carious teeth, as they can be an additional and undesirable source of infection. The anesthesiologist finds out and evaluates the psychoneurological state of the patient. So, for example, in schizophrenia, the use of hallucinogenic drugs (ketamine) is contraindicated. Surgery during the period of psychosis is contraindicated. In the presence of a neurological deficit, it is preliminarily corrected. Allergic history is of great importance for the anesthesiologist, for this, intolerance to drugs, as well as food, household chemicals, etc. is specified. If the patient has a aggravated allergic anamnesis, not even to medications, during anesthesia, an allergic reaction can develop up to anaphylactic shock. Therefore, desensitizing agents (diphenhydramine, suprastin) are introduced into premedication in large quantities. An important point is the presence of a patient in the past operations and anesthesia. It turns out what the anesthesia was and whether there were any complications. Attention is drawn to the somatic condition of the patient: the shape of the face, the shape and type of the chest, the structure and length of the neck, the severity of subcutaneous fat, the presence of edema. All this is necessary in order to choose the right method of anesthesia and drugs. The first rule for preparing a patient for anesthesia during any operation and when using any anesthesia is the cleansing of the gastrointestinal tract (the stomach is washed through the tube, cleansing enemas are performed). To suppress the psycho-emotional reaction and suppress the activity of the vagus nerve, before surgery, the patient is given medication - premedication. At night, phenazepam is prescribed intramuscularly. Patients with a labile nervous system are prescribed tranquilizers (seduxen, relanium) a day before surgery. 40 minutes before surgery, narcotic analgesics are administered intramuscularly or subcutaneously: 1 ml of a 1-2% solution of promolol or 1 ml of pentozocine (lexir), 2 ml of fentanyl, or 1 ml of 1% morphine. To suppress the function of the vagus nerve and reduce salivation, 0,5 ml of a 0,1% solution of atropine is administered. Immediately before the operation, the oral cavity is examined for the presence of removable teeth and prostheses that are removed. 3. Intravenous anesthesia The advantages of intravenous general anesthesia are the rapid introduction of the patient into anesthesia. With this type of anesthesia, there is no excitement, and the patient quickly falls asleep. But narcotic drugs that are used for intravenous administration create short-term anesthesia, so they cannot be used in their pure form as mononarcosis for long-term operations. Barbiturates - sodium thiopental and hexenal - are able to quickly induce narcotic sleep, while there is no stage of excitation, and awakening is fast. Clinical pictures of anesthesia conducted by sodium thiopental and hexenal are similar. Geksenal has a less inhibitory effect on the respiratory center. Freshly prepared solutions of barbituric acid derivatives are used. The contents of the vial (1 g of the drug) are dissolved before the onset of anesthesia in 100 ml of isotonic sodium chloride solution (1% solution). The peripheral or central (according to indications) vein is punctured and the prepared solution is slowly injected at a rate of 1 ml for 10-15 s. When the solution was injected in a volume of 3-5 ml, the patient's sensitivity to barbituric acid derivatives is determined within 30 seconds. If no allergic reaction is noted, then continue the introduction of the drug until the surgical stage of anesthesia. Since the onset of narcotic sleep, with a single injection of anesthetic, the duration of anesthesia is 10-15 minutes. To maintain anesthesia, barbiturates are administered in fractions of 100-200 mg of the drug, up to a total dose of not more than 1 g. During the administration of barbiturates, the nurse keeps a record of the pulse, blood pressure and respiration. The anesthesiologist monitors the state of the pupil, the movement of the eyeballs, the presence of a corneal reflex to determine the level of anesthesia. Anesthesia with barbiturates, especially thiopental-sodium, is characterized by depression of the respiratory center, so the presence of an artificial respiration apparatus is necessary. When respiratory arrest (apnea) occurs, artificial lung ventilation (ALV) is performed using a mask of a breathing apparatus. Rapid administration of thiopental sodium can lead to a decrease in blood pressure and cardiac depression. In this case, the administration of the drug is stopped. In surgery, anesthesia with barbiturates as mononarcosis is used for short-term operations that do not exceed 20 minutes in duration (for example, opening abscesses, phlegmon, reduction of dislocations, diagnostic manipulations, and repositioning of bone fragments). Derivatives of barbituric acid are also used for induction anesthesia. Viadryl (predion for injection) is used at a dose of 15 mg/kg, with a total dose of 1000 mg on average. Viadryl is mainly used in small doses along with nitrous oxide. In high doses, this drug may cause a decrease in blood pressure. A complication of its use is the development of phlebitis and thrombophlebitis. In order to prevent their development, it is recommended to administer the drug slowly into the central vein in the form of a 2,5% solution. Viadryl is used for endoscopic examinations as an introductory type of anesthesia. Propanidide (epontol, sombrevin) is available in ampoules of 10 ml of a 5% solution. The dose of the drug is 7-10 mg / kg, administered intravenously, quickly (the entire dose is 500 mg in 30 seconds). Sleep comes immediately - "at the end of the needle." The duration of anesthesia sleep is 5-6 minutes. Awakening is fast, calm. The use of propanidide causes hyperventilation, which occurs immediately after loss of consciousness. Apnea may sometimes occur. In this case, ventilation should be carried out using a breathing apparatus. The negative side is the possibility of hypoxia formation against the background of the drug administration. It is necessary to control blood pressure and pulse. The drug is used for induction anesthesia in outpatient surgical practice for small operations. Sodium hydroxybutyrate is administered intravenously very slowly. The average dose is 100-150 mg/kg. The drug creates a superficial anesthesia, so it is often used in combination with other narcotic drugs, such as barbiturates - propanidide. It is often used for induction anesthesia. Ketamine (ketalar) can be used for intravenous and intramuscular administration. The estimated dose of the drug is 2-5 mg / kg. Ketamine can be used for mononarcosis and for induction anesthesia. The drug causes superficial sleep, stimulates the activity of the cardiovascular system (blood pressure rises, pulse quickens). The introduction of the drug is contraindicated in patients with hypertension. Widely used in shock in patients with hypotension. Side effects of ketamine can be unpleasant hallucinations at the end of anesthesia and upon awakening. 4. Inhalation anesthesia Inhalation anesthesia is carried out with the help of easily evaporating (volatile) liquids - ether, halothane, methoxy-flurane (pentran), trichlorethylene, chloroform or gaseous narcotic substances - nitrous oxide, cyclopropane. With the endotracheal method of anesthesia, the narcotic substance enters the body from the anesthesia machine through a tube inserted into the trachea. The advantage of the method lies in the fact that it provides free patency of the respiratory tract and can be used in operations on the neck, face, head, eliminates the possibility of aspiration of vomit, blood; reduces the amount of drug used; improves gas exchange by reducing "dead" space. Endotracheal anesthesia is indicated for major surgical interventions, it is used as a multicomponent anesthesia with muscle relaxants (combined anesthesia). The total use of several drugs in small doses reduces the toxic effects on the body of each of them. Modern mixed anesthesia is used to provide analgesia, turn off consciousness, relaxation. Analgesia and switching off consciousness are carried out by using one or more narcotic substances - inhaled or non-inhaled. Anesthesia is carried out at the first level of the surgical stage. Muscle relaxation, or relaxation, is achieved by the fractional administration of muscle relaxants. 5. Stages of anesthesia There are three stages of anesthesia. 1. Introduction to anesthesia. Introductory anesthesia can be carried out with any narcotic substance, against which a rather deep anesthetic sleep occurs without a stage of excitation. Mostly, barbiturates, fentanyl in combination with sombrevin, milled with sombrevin are used. Sodium thiopental is also often used. The drugs are used in the form of a 1% solution, they are administered intravenously at a dose of 400-500 mg. Against the background of induction anesthesia, muscle relaxants are administered and tracheal intubation is performed. 2. Maintenance of anesthesia. To maintain general anesthesia, you can use any narcotic that can protect the body from surgical trauma (halothane, cyclopropane, nitrous oxide with oxygen), as well as neuroleptanalgesia. Anesthesia is maintained at the first and second levels of the surgical stage, and to eliminate muscle tension, muscle relaxants are administered, which cause myoplegia of all skeletal muscle groups, including respiratory ones. Therefore, the main condition for the modern combined method of anesthesia is mechanical ventilation, which is carried out by rhythmically squeezing a bag or fur, or using an artificial respiration apparatus. Recently, the most widespread neuroleptanalgesia. With this method, nitrous oxide with oxygen, fentanyl, droperidol, muscle relaxants are used for anesthesia. Introductory anesthesia intravenous. Anesthesia is maintained by inhalation of nitrous oxide with oxygen in a ratio of 2: 1, fractional intravenous administration of fentanyl and droperidol 1-2 ml every 15-20 minutes. With increased heart rate, fentanyl is administered, with an increase in blood pressure - droperidol. This type of anesthesia is safer for the patient. Fentanyl enhances pain relief, droperidol suppresses vegetative reactions. 3. Withdrawal from anesthesia. By the end of the operation, the anesthesiologist gradually stops the administration of narcotic substances and muscle relaxants. Consciousness returns to the patient, independent breathing and muscle tone are restored. The criterion for assessing the adequacy of spontaneous breathing are indicators of RO2, RSO2, pH. After awakening, restoration of spontaneous breathing and skeletal muscle tone, the anesthesiologist can extubate the patient and transport him for further observation in the recovery room. 6. Methods for monitoring the conduct of anesthesia During general anesthesia, the main parameters of hemodynamics are constantly determined and evaluated. Measure blood pressure, pulse rate every 10-15 minutes. In persons with diseases of the cardiovascular system, as well as in thoracic operations, it is necessary to constantly monitor the function of the heart muscle. Electroencephalographic observation can be used to determine the level of anesthesia. To control lung ventilation and metabolic changes during anesthesia and surgery, it is necessary to conduct a study of the acid-base state (RO2, RSO2, pH, BE). During anesthesia, the nurse maintains an anesthetic chart of the patient, in which she necessarily records the main indicators of homeostasis: pulse rate, blood pressure, central venous pressure, respiratory rate, and ventilator parameters. In this map, all stages of anesthesia and surgery are fixed, the doses of narcotic substances and muscle relaxants are indicated. All drugs used during anesthesia are noted, including transfusion media. The time of all stages of the operation and the administration of drugs is recorded. At the end of the operation, the total number of all the means used is indicated, which is also reflected in the anesthesia card. A record is made of all complications during anesthesia and surgery. The anesthesia card is embedded in the medical history. 7. Complications of anesthesia Complications during anesthesia may occur due to improper anesthesia technique or the effect of anesthetics on vital organs. One such complication is vomiting. At the beginning of the introduction of anesthesia, vomiting may be associated with the nature of the dominant disease (pyloric stenosis, intestinal obstruction) or with the direct effect of the drug on the vomiting center. Against the background of vomiting, aspiration is dangerous - the entry of gastric contents into the trachea and bronchi. Gastric contents that have a pronounced acid reaction, falling on the vocal cords and then penetrating the trachea, can lead to laryngospasm or bronchospasm, resulting in respiratory failure with subsequent hypoxia - this is the so-called Mendelssohn's syndrome, accompanied by cyanosis, bronchospasm, tachycardia. Dangerous can be regurgitation - passive throwing of gastric contents into the trachea and bronchi. This usually occurs against the background of deep anesthesia using a mask with relaxation of the sphincters and overflow of the stomach or after the introduction of muscle relaxants (before intubation). Ingestion into the lung during vomiting or regurgitation of acidic gastric contents leads to severe pneumonia, often fatal. In order to avoid the appearance of vomiting and regurgitation, it is necessary to remove its contents from the stomach with a probe before anesthesia. In patients with peritonitis and intestinal obstruction, the probe is left in the stomach during the entire anesthesia, while a moderate Trendelenburg position is necessary. Before the onset of anesthesia, to prevent regurgitation, you can apply the Selick method - pressure on the cricoid cartilage posteriorly, which causes clamping of the esophagus. If vomiting occurs, it is necessary to quickly remove the gastric contents from the oral cavity with a swab and suction; in case of regurgitation, the gastric contents are removed by suction through a catheter inserted into the trachea and bronchi. Vomiting followed by aspiration can occur not only during anesthesia, but also when the patient wakes up. To prevent aspiration in such cases, it is necessary for the patient to take a horizontal position or the Trendelenburg position, turn his head to the side. The patient should be monitored. Complications from the respiratory system can occur due to impaired airway patency. This may be due to defects in the anesthesia machine. Before starting anesthesia, it is necessary to check the functioning of the device, its tightness and the permeability of gases through the breathing hoses. Airway obstruction may occur as a result of retraction of the tongue during deep anesthesia (level III of the surgical stage of anesthesia). During anesthesia, solid foreign bodies (teeth, prostheses) can enter the upper respiratory tract. To prevent these complications, it is necessary to advance and support the lower jaw against the background of deep anesthesia. Before anesthesia, the dentures should be removed, the patient's teeth should be examined. Complications of tracheal intubation performed by direct laryngoscopy can be grouped as follows: 1) damage to the teeth by the laryngoscope blade; 2) damage to the vocal cords; 3) introduction of an endotracheal tube into the esophagus; 4) introduction of an endotracheal tube into the right bronchus; 5) exit of the endotracheal tube from the trachea or bending it. The described complications can be prevented by a clear knowledge of the intubation technique and control of the position of the endotracheal tube in the trachea above its bifurcation (using auscultation of the lungs). Complications from the circulatory system. A decrease in blood pressure both during the period of anesthesia and during anesthesia can occur due to the effect of narcotic substances on the activity of the heart or on the vascular-motor center. This happens with an overdose of narcotic substances (often halothane). Hypotension may appear in patients with low BCC with the optimal dosage of narcotic substances. To prevent this complication, it is necessary to fill the BCC deficit before anesthesia, and during the operation, accompanied by blood loss, transfuse blood-substituting solutions and blood. Heart rhythm disturbances (ventricular tachycardia, extrasystole, ventricular fibrillation) can occur due to a number of reasons: 1) hypoxia and hypercapnia resulting from prolonged intubation or insufficient ventilation during anesthesia; 2) overdose of narcotic substances - barbiturates, halothane; 3) the use of epinephrine against the background of halothane, which increases the sensitivity of halothane to catecholamines. Electrocardiographic control is needed to determine the heart rhythm. Treatment is carried out depending on the cause of the complication and includes the elimination of hypoxia, a decrease in the dose of the drug, the use of quinine drugs. Cardiac arrest is the most dangerous complication during anesthesia. The reason for it is most often incorrect control over the patient's condition, errors in the technique of anesthesia, hypoxia, hypercapnia. Treatment consists of immediate cardiopulmonary resuscitation. Complications from the nervous system. During general anesthesia, a moderate decrease in body temperature is allowed as a result of the influence of narcotic substances on the central mechanisms of thermoregulation and cooling of the patient in the operating room. The body of patients with hypothermia after anesthesia tries to restore body temperature due to increased metabolism. Against this background, at the end of anesthesia and after it, chills appear, which is observed after halothane anesthesia. To prevent hypothermia, it is necessary to monitor the temperature in the operating room (21-22 ° C), cover the patient, if necessary, infusion therapy, pour solutions warmed to body temperature, and inhale warm, moistened narcotic drugs. Cerebral edema is a consequence of prolonged and deep hypoxia during anesthesia. Treatment should be immediate, it is necessary to follow the principles of dehydration, hyperventilation, local cooling of the brain. Peripheral nerve damage. This complication occurs a day or more after anesthesia. Most often, the nerves of the upper and lower extremities and the brachial plexus are damaged. This is the result of an incorrect position of the patient on the operating table (abduction of the arm more than 90° from the body, placing the arm behind the head, fixing the arm to the arc of the operating table, laying the legs on holders without padding). The correct position of the patient on the table eliminates the tension of the nerve trunks. Treatment is carried out by a neuropathologist and a physiotherapist. Lecture number 13. Local anesthesia 1. Surface anesthesia This type of anesthesia is carried out by contact of the anesthetic substance with one or another organ, its surface. For this purpose, a 1-3% solution of cocaine, a 0,25-2% solution of dicaine, a 1-2% solution of lidocaine, a 1-5% solution of trimecaine and a 0,5-2% solution of pyromecaine solution. Most local anesthetics bind to inactivated sodium channels, preventing their activation and sodium entry into the cell during membrane depolarization, thus achieving an analgesic effect. The technique of surface anesthesia is simple and consists in lubricating, instilling a solution or spraying it using special spray guns. The onset of action of the anesthetic is pH dependent, with low pH taking longer to take effect than at high pH. The duration of action of the anesthetic depends on the degree of its binding to proteins. This type of anesthesia is used in diagnostic manipulations and in ophthalmology, otorhinolaryngology. 2. Regional anesthesia Regional anesthesia includes plexus, conduction, epidural, paravertebral and other types of anesthesia. Unlike general anesthesia, regional anesthesia provides adequate surgical analgesia due to peripheral blockade of pain impulses while maintaining normal vital functions. Regional anesthesia is technically difficult, and requires accurate knowledge of the anatomical and topographic location of the nerve plexus or nerve conductor, a clear orientation in permanent identification points (bone protrusions, arteries, muscles), the ability to assess tissue resistance and patient sensations. To turn off pain sensitivity, it is enough to introduce a 1% solution of trimecaine (lidocaine), and to turn off proprioceptive sensitivity and achieve muscle relaxation, you need to use more concentrated local anesthetic solutions (for example, trimecaine 2-2,5%). The restoration of sensitivity goes in the reverse order, i.e., first muscle tone and proprioceptive sensations appear, and then pain and temperature sensations. It is important to take into account that with an increase in the concentration and amount of local anesthetic, its toxicity increases. The most widely used solutions are trimecaine 1-2%, lidocaine 1-2% and bupivocaine 0,75-0,5%. Local anesthetics block myelin-free and thin myelin fibers more easily and quickly (all vegetative, as well as conducting temperature and pain stimuli). The thick myelin fibers going to the skeletal muscles, tactile receptors and proprioceptors are highly resistant to anesthesia, since they are affected by local anesthesia in the area of Ranvier's intercepts. Diffusion of the anesthetic into the lipoid part of the nerve fiber is rapid, but only until the concentration outside the nerve is higher than in the nerve itself. After this ratio changes, the anesthetic diffuses in the opposite direction from the nerve into the surrounding tissues. Weakly concentrated anesthetic solutions, administered in large volumes, spread widely, but their diffusion is negligible. Concentrated solutions introduced in small volumes have a good degree of diffusion. The effect of anesthesia depends on the amount of anesthetic penetrating transperineurally and causing an adequate threshold block. Doubling the concentration of the injected anesthetic prolongs anesthesia by 1/3, and the introduction of a double volume - only by 3-9%. Local anesthetics often lead to anaphylactic reactions. Trimecaine: duration of action is 1-1,5 hours, the maximum single dose is 800-1000 mg. Lidocaine (xicaine) is used in a 1-2% solution, the duration of anesthesia is up to 2,5-3 hours. Bupivocaine (marcaine) used in a 0,5-0,75% solution in a maximum single dose of 150-170 mg, duration of action 8-12 hours. For the use of long-acting anesthetics, the addition of lidocaine clearly accelerates the onset of the effect, reducing the latent period. When conducting regional anesthesia, you need to know and follow the general rules: 1) clearly know the anatomical and topographic features of the nerve plexuses and conductors in the area of the proposed anesthesia, as well as the technique of performing anesthesia; 2) choose the right local anesthetic, determine its concentration, total dose and method of delivery to the nerve plexus or conductor; 3) assess the patient's condition and find out the allergic and pharmacotherapeutic background; 4) warn the patient about the possible preservation of deep tactile and proprioceptive sensitivity during conduction and plexus anesthesia; 5) to constantly monitor the patient's hemodynamics and respiration after anesthesia; 6) before or immediately after anesthesia, perform venipuncture and take measures to prevent possible complications; 7) carry out in compliance with aseptic and antiseptic measures, and carefully remove chemically active substances (iodine, chlorhexidine, etc.) from the skin surface before puncture; 8) before the introduction of a local anesthetic, it is imperative to conduct an aspiration test to prevent the needle from entering the arterial vessel; 9) remember that the paresthesia felt by the patient during regional anesthesia is a prerequisite for the success of obtaining analgesia; its absence indicates a possible failure; 10) to facilitate the finding of the nerve conductor or plexus, it is advisable to apply electrical stimulation by applying a negative impulse to the needle, and a positive impulse (anode) to the indifferent pole of the electrode, which is fixed on the patient's skin. 3. Anesthesia of the cervical plexus (ASP) ACS, performed on one or both sides, allows you to perform all operations on the neck, thyroid gland, brachiocephalic vessels in case of gunshot wounds, injuries and tumor diseases. The cervical plexus (Plexus cervicalis) is formed from the anterior branches of the four upper cervical nerves (C1-FROM4) at their exit from the intervertebral foramina. It is located on the anterior surface of the middle scalene muscle and the muscle that lifts the scapula, lateral to the transverse processes of the cervical vertebrae. The motor nerves of the cervical plexus innervate the muscles of the neck, and the sensory nerves innervate the skin of the occipital region of the head, the anterior and lateral surfaces of the neck, the subclavian region to the level of I and II ribs and the auricle. The largest nerve of the cervical plexus is the phrenic (p. prenicus), which is formed from C3-FROM4 and less often due to an additional branch from C5. Most sensory nerves exit in the middle from under the posterior edge of the sternocleidomastoid muscle and diverge in the superficial layers of the neck, skin of the occipital region of the head and upper chest. When anesthesia of the sensitive nerves of the cervical plexus along the posterior edge in the middle of the sternocleidomastoid muscle, a number of operations on the neck can be performed, but it should be remembered that anesthesia must be bilateral, since the nerves anastomose along the midline of the neck. For large operations in the deep layers of the neck (strumectomy, removal of neck tumors, laryngectomy, carotid endarterectomy, etc.), it is necessary to anesthetize the cervical plexus with an anterior approach. With lateral access, there is a risk of serious complications (introduction of an anesthetic solution into the subdural space), so it is not used. To perform an ASS with an anterior approach, it is necessary that the patient lie on his back with a small roll under the neck. The head should lie flat, straight in the midline without turning or be slightly turned in the direction opposite to the puncture. Hands along the body, the anesthesiologist stands at the head of the puncture. Landmarks: sternocleidomastoid muscle, hyoid bone, internal carotid artery and mandibular angle. Anesthesia technique 2 cm below the angle of the lower jaw, anterior to the sternocleidomastoid muscle, the pulsation of the internal carotid artery is determined. The horizontal branch of the hyoid bone corresponds to the level of the transverse process of vertebra C3. The needle injection point is located at the intersection of the line, which is a continuation of the horizontal branch of the hyoid bone, with the anterior edge of the sternocleidomastoid muscle. At this point of intersection, under aseptic conditions, a “lemon peel” is formed, and an injection needle is directed through it from the outside to the inside and from front to back, advancing it medially to the sternocleidomastoid muscle and behind the internal carotid artery (in the gap between the sternocleidomastoid muscle and palpable internal carotid artery) until the patient develops paresthesia or the needle hits the transverse process of vertebra C3. The depth of the injection does not exceed 2-5 cm. The needle is securely fixed in this position and an aspiration test is performed, determining whether the end of the needle is in the lumen of the vessel. For anesthesia, 10-12 ml of a 2% solution of trimecaine is injected from both sides. To enhance the anesthesia of the cervical plexus, you can additionally block the superficial branches that go to the anterior surface of the neck. The place of their exit is the middle of the posterior edge of the sternocleidomastoid muscle. The needle is injected at the point of their exit under the superficial fascia of the neck. A solution of 2% trimecaine is administered in an amount of 3-5 ml cranially and caudally (fan-shaped). Adequate anesthesia occurs after 8-12 minutes and provides effective pain relief in most patients within 1,5-2 hours. Complications Horner's syndrome, phrenic nerve block, recurrent nerve paresis, hypotension with intravascular injection of anesthetic. Hoarseness is the main symptom of cervical plexus block. The most dangerous complication with lateral access is the development of high spinal anesthesia and the ingress of anesthetic into the cavity of the IV ventricle, the symptoms of which are paralysis of the bulbar centers, pupil dilation, lowering blood pressure, respiratory failure and muscle atony. Absolute contraindications to anesthesia of the cervical plexus are paresis of the phrenic nerve on the opposite side, damage to the nerves of the cervical plexus. 4. Anesthesia of the brachial plexus (APS) APS allows you to perform all operations on the upper limb, shoulder joint, shoulder, forearm and hand: amputations, surgical treatment of wounds with reposition and fixation of bone fragments, operations on blood vessels and nerves, reduction of shoulder dislocation, etc. High frequency of surgical interventions on the upper limb, especially in wartime, raises the question of rational methods of anesthesia during these operations. Topographically, there are 2 parts of the brachial plexus: supraclavicular and subclavian. Branches extend from the supraclavicular part to the deep muscles of the neck and muscles of the shoulder girdle. The subclavian part of the brachial plexus consists of three trunks covering the axillary artery from the inner, back and outer sides. Long nerves originate from the trunks, going to the free part of the upper limb, and one short nerve to the shoulder girdle. From the inner trunk depart the ulnar nerve and the lower root to the median nerve; the radial and axillary nerves depart from the posterior trunk, and the musculocutaneous nerve and the upper root of the median nerve depart from the external trunk. Therefore, anesthesia of the brachial plexus is possible in various ways using supraclavicular, axillary and subclavian approaches. Of the supraclavicular approaches, the methods of anesthesia in the interstitial space are most widely used as the most simple, reliable and having fewer complications. Brachial plexus anesthesia by Winnie The patient lies on his back, his head is turned in the opposite direction from the puncture site, the chin is brought to the contralateral shoulder girdle. The hand from the side of the puncture lies along the body, slightly pulled down. Landmarks: sternocleidomastoid muscle, scalenus anterior, interstitial space, external jugular vein, clavicle, cricoid cartilage. Anesthesia technique. The skin is treated with an antiseptic solution. Behind the sternocleidomastoid muscle, which clearly contours with a slight rise of the head, at the level of the cricoid cartilage, the fingertips of the left hand are placed on the anterior scalene muscle. With further displacement of the fingers laterally by 0,5-1,5 cm between the anterior and middle scalene muscles, the interscalene gap is probed. It becomes more distinct with a deep breath, as this tenses the scalene muscles. In the depth of the interstitial space, the transverse processes of the cervical vertebrae are palpated (feeling of firm resistance), and with increased pressure with the tip of the finger, paresthesia in the shoulder or shoulder girdle is often caused; more caudally, in the interstitial space, the subclavian artery can be palpated. The interstitial space in the upper section is crossed by the external jugular vein. The needle injection point is located in the upper part of the interstitial space at the level of the cricoid cartilage. At this point, a "lemon crust" is formed, and through it the needle is directed medially and somewhat downward, from front to back (in the dorsal direction) to the transverse process C6 at an angle of 30° to the sagittal plane. When the needle is advanced inward at a distance of 1,5-4 cm, paresthesia occurs, and the tip of the needle rests on the transverse process of the 6th cervical vertebra. In this position, the needle is fixed or pulled up by 1-2 mm, and after the aspiration test, 30-40 ml of a 2% solution of trimecaine (lidocaine) or a 0,5-0,75% solution of bupivocaine (marcaine) is injected. During the introduction of the first milliliters of local anesthetic solution, the patient experiences a short-term pain ("electric shock"), indicating the correct location of the needle tip. In the absence of paresthesia, the position of the needle tip can be checked by introducing 0,5 ml of a 0,9% sodium chloride solution taken from the refrigerator. The appearance of a feeling of ache in the upper limb indicates the contact of a cold solution with a nerve. Contraindications: paresis of the recurrent or phrenic nerve on the opposite side, damage to the nerves of the brachial plexus. Possible complications: intravascular injection of a local anesthetic solution, especially into the vertebral artery, the rapid diffusion of which leads to CNS intoxication; subarachnoid administration of an anesthetic solution causes a total spinal block; epidural administration results in high epidural anesthesia. Anesthesia of the brachial plexus in the interstitial space according to the method of S. V. Gavrilin and L. G. Tikhonov The main difference of this method from others is that there are no dome of the pleura and large blood vessels at the puncture site. The patient lies on his back, his head is turned in the opposite direction from the puncture site, the chin is brought to the contralateral shoulder girdle. A small roller is placed under the shoulders, the arm from the side of the puncture lies along the body. Landmarks: clavicle, sternocleidomastoid muscle. Anesthesia technique. The needle injection point is located at the top of the perpendicular, restored from the upper edge of the middle of the clavicle, the length of which is equal to ¼ of the length of the sternocleidomastoid muscle. At this point, a “lemon peel” is formed, the intramuscular injection needle is inserted at an angle of 60 ° to the skin surface, while the needle and an imaginary or drawn perpendicular should be in the same plane. The needle is inserted in the direction of the transverse process of the 6th cervical vertebra until paresthesia appears in the upper limb. In the absence of paresthesia, the needle is advanced all the way into the transverse process of the 6th cervical vertebra, and after pulling it towards itself by 1-2 mm, 30-40 ml of a 2% solution of trimecaine or lidocaine is injected. The needle insertion depth is 2-5 cm. Anesthesia of the brachial plexus in the modification of V. S. Sokolovsky The patient lies on his back, the head is located in the midline, the arms lie along the body. Landmarks: sternocleidomastoid muscle and clavicle. Anesthesia technique. To facilitate finding a point on the skin of the neck, a triangle is built with a vertex in the area of the sternoclavicular joint on the side of anesthesia. The rays of the triangle are the axis of the clavicle and a straight line connecting the mastoid process with the sternoclavicular joint. A perpendicular is restored to the bisector of the angle ABC from the middle of the clavicle. The point of intersection is the point of injection of the needle, which advances at an angle of 45° relative to the horizontal plane of the operating table and perpendicular to the axis of the cervical spine. At a depth of 2-3 cm, after receiving paresthesia and conducting an aspiration test, 30-40 ml of a 2% solution of trimecaine (lidocaine) is injected. Anesthesia occurs in 10-12 minutes. When inconclusive paresthesia is obtained, it is desirable to use electrical stimulation to identify the nerve trunk of the cervical plexus. 5. Anesthesia of peripheral nerves in the wrist area For operations on the hand, it is necessary to anesthetize the ulnar, median and radial nerves. In all cases, the needle is injected at the level of the proximal fold of the wrist. During anesthesia, the patient lies on his back, in the area of \uXNUMXb\uXNUMXbthe wrist, the arm is supinated and slightly bent. Landmarks: Ulna styloid, pisiform, flexor carpi ulnaris tendon, and flexor carpi longus tendon. Anesthesia of the ulnar nerve Topography of the ulnar nerve. In the lower third of the forearm, the ulnar nerve runs lateral to the flexor ulnar tendon and medial to the ulnar artery. At the level of the wrist or on the flexor surface of the forearm, 3-5 cm proximal to the wrist, the ulnar nerve divides into two branches - dorsal and palmar. The dorsal branch is sensitive, goes under the tendon of the ulnar flexor of the hand and exits into the subcutaneous tissue of the rear of the hand at approximately the level of the wrist joint. Anastomosing with the branches of the radial nerve, it innervates 2 ½ fingers. The palmar branch of the ulnar nerve is mixed and at the level of the pisiform bone it is divided into two branches - deep and superficial. The latter is sensitive and innervates the hypothenar region of the 5th finger and the ulnar side of the ring finger. Technique for anesthesia of the palmar branch of the ulnar nerve. The point of injection of the needle is at the level of the proximal fold of the wrist medial to the tendon of the ulnar flexor of the hand. The needle to a depth of 1-2 cm is passed through the subcutaneous tissue towards the pisiform bone. After the appearance of paresthesia and with a negative aspiration test, the needle is fixed and 3-5 ml of a 2% trimecaine solution is injected. In the absence of paresthesia, the needle is advanced until it comes into contact with the bone, and when it is removed, the tissues are infiltrated with a 2% trimekia solution. Technique for anesthesia of the dorsal branch of the ulnar nerve. The point of injection of the needle is at the level of the intersection of the proximal fold of the wrist with the medial edge of the tendon of the flexor ulnaris muscle. The needle is directed to the styloid process of the ulna. To obtain paresthesia, 3-5 ml of a 2% solution of trimecaine is injected. In the absence of paresthesia, the needle is removed, and 5-10 ml of a 2% trimecaine solution is infiltrated into the tissues. There are no complications. median nerve anesthesia The patient lies on his back, the arm is supinated and straightened. Landmarks: tendon of the long palmar muscle, tendon of the radial flexor of the hand and proximal skin fold of the wrist. Topography. In the lower third of the forearm, the median nerve passes in the medial groove very close to the surface of the skin, located under the fascia, and approximately 4-5 cm above the distal skin fold. The medial groove is formed from the outside by the tendon of the radial flexor of the hand, on the ulnar side - by the tendon of the long flexor of the hand. The branches of the median nerve innervate the 1st, 2nd, 3rd fingers and the outer surface of the 4th finger, as well as the thenar muscles. Anesthesia technique. The needle injection point is located on the line of the proximal skin fold of the wrist between the tendon of the long palmar muscle and the radial flexor of the hand. After perpendicular advancement of the needle through the subcutaneous tissue to a depth of 0,5-1 cm and obtaining paresthesia, the needle is fixed and 3-5 ml of a 2% lidocaine solution is injected. If it is not possible to achieve paresthesia at a depth of 1 cm, the tissues are fan-shaped infiltrated with 5-10 ml of a 2% trimecaine solution while slowly withdrawing the needle. Anesthesia of the radial nerve Landmarks: styloid process of the radius, radial artery, "anatomical snuffbox". Topography. The superficial branch of the radial nerve first goes to the forearm along with the radial artery, and then in the lower third of the forearm at a distance of about 8 cm from the wrist joint, the nerve crosses with the tendon of the brachioradialis muscle and passes to the posterior surface of the forearm, where it goes distally and posteriorly, crossing the long abductor of the large finger and its short extensor. At the level of the wrist at the top of the "anatomical snuffbox" it divides into terminal branches that innervate fingers 1, 2 and the outer surface of fingers 3. The branches of all three nerves often anastomose with each other at the forearm, wrist, and hand. Anesthesia technique. The injection point is located at the level of the proximal skin fold of the wrist lateral to the radial artery on the projection of the top of the "anatomical snuffbox". It is injected, and the needle is directed towards the "snuffbox". When paresthesia occurs, the needle is fixed, with a negative aspiration test, 3-5 ml of a 2% trimecaine solution is injected. In the absence of paresthesia, 5-10 ml of a 2% solution of trimecaine is fan-shapedly injected into the underlying tissues, creating an infiltration "bracelet" 3-3,5 cm long from the tendons of the short extensor and long abductor of the thumb on one side, to the long extensor of the thumb - with another. 6. Anesthesia of the lower extremities To perform surgical interventions on the lower limb, it is necessary to anesthetize all four major nerves. Three of them - the femoral, obturator and external cutaneous nerve of the thigh - originate from the lumbar plexus, and the sciatic nerve is formed partly from the lumbar and three branches of the sacral plexus. Each nerve innervates certain areas of the lower limb: the femoral nerve - the anterior surface of the thigh, the anterior inner surface of the lower leg and rear of the foot; ischial - rear and lateral surface of the lower leg, plantar surface of the foot and its outer edge, obturator - inner surface of the thigh; external cutaneous nerve of the thigh - the lateral surface of the thigh. Zones of deep sensitivity coincide with the innervation of the skin zones. All muscles of the lower limb receive motor fibers from the femoral and sciatic nerves, except for the group of abductors innervated by the obturator nerve. The knee joint and its anterior surface are innervated by the femoral nerve, the inner surface by the obturator, the lateral by the external cutaneous and sciatic, and the posterior by the posterior cutaneous, femoral and sciatic nerves. Anesthesia of the lumbar plexus by anterior (inguinal) access The patient lies on his back. Landmarks: inguinal ligament and femoral artery. Topography. The lumbar plexus is formed by the anastomosing anterior branches of the L roots.1-L2-L3 and partly by the anterior branches of Th12 and L4 nerves. The plexus is located in front of the transverse processes of the lumbar vertebrae between the square muscle of the lower back - dorsally, the psoas major muscle - ventrally, the bodies of the vertebrae - medially, gives off muscle branches and goes down in the fascial bed. The lumbar spinal nerves have connecting branches with the lumbar nodes of the sympathetic trunks, contain motor, sensory and sympathetic nerve fibers. The largest nerves of the lumbar plexus are the external femoral cutaneous nerve (L2-L3), obturator (L2-L4) and femoral (L2-L4) nerves. The latter is a continuation of the lumbar plexus, extends to the thigh under the pupart ligament through the muscular lacuna, being separated from the medially located femoral artery by the iliopectineal ligament. The width of the femoral nerve in this place is about 0,7-0,8 cm. The depth of the femoral nerve is on average 1,8-3 cm. -0,5 cm. Below the inguinal ligament, the nerve forms two bundles. The branches of the anterior bundle innervate the quadriceps femoris muscle, the middle and medial side of the knee joint and pass into the saphenous nerve, which innervates the medial surface of the lower leg and the inner ankle. Anesthesia of the sciatic nerve makes it possible to operate in any area below the knee joint, to reduce all fractures of the bones of the lower limb, excluding a fracture of the femoral neck. Anesthesia technique. The skin is treated with a disinfectant solution. A "lemon peel" is formed with an anesthetic solution, after which the needle is injected under the inguinal ligament 1-1,5 cm below the latter and 0,5-1 cm lateral to the palpable femoral artery. The needle is directed through the subcutaneous tissue somewhat in the proximal direction under the inguinal ligament, where at a depth of 3-4 cm after puncture of the fascia, the needle fails with loss of resistance and paresthesia may occur, extending to the anterior surface of the thigh. In this position, the needle is fixed with the thumb and forefinger of the left hand, and the edge of the palm of the left hand is pressed with force on the soft tissues of the thigh distal to the needle and 35-40 ml of a 1,5% trimecaine solution is injected. Pressure on soft tissues lasts 1,5-2 minutes. Thus, anesthesia of the femoral nerve with clamping turns into anesthesia of the lumbar plexus, performed from the anterior approach. The action of anesthesia lasts 2-2,5 hours. Complications: rather rapid resorption of the anesthetic solution is possible when anesthesia of the lumbar plexus is combined with anesthesia of the sciatic nerve, when the total single dose exceeds 1 g of the drug. Anesthesia of the lumbar plexus can be performed by the posterior approach, while simultaneously anesthetizing the femoral (L2-L4) and obturator nerves (L2-L3), genitofemoral nerve (L1-L2) and the lateral femoral cutaneous nerve (L2-L3). The position of the patient during anesthesia is on a healthy side with bent legs. Landmarks: spinous process of the 4th lumbar vertebra (line connecting the iliac crests behind); 3 cm caudal along the crests of the spinous processes from the spinous process of the 4th lumbar vertebra and 5 cm lateral from the last point. Anesthesia technique. From the spinous process of the 4th lumbar vertebra in the caudal direction, a line 3 cm long is drawn, from the end of which a perpendicular 5 cm long is laterally restored upward. The end point of the perpendicular, lying near the iliac crest, is the needle injection point. After creating a "lemon peel", a 12-15 cm long needle is inserted perpendicular to the skin until it contacts the transverse process of the 5th lumbar vertebra. Cranially, sliding off the transverse process of the 5th lumbar vertebra, the needle enters the thickness of the square psoas muscle. At the same time, resistance is felt to the introduction of a solution into it with a syringe (or springy resistance occurs with deformation of the air bubble in the syringe). The needle is passed to a depth where there is a feeling of "loss of resistance" (or the air bubble is not deformed). This test indicates that the needle is in the fascia between the quadrate psoas and psoas major. The needle is fixed at this depth and 35-40 ml of a 1,5-2% trimecaine (lidocaine) solution is injected to achieve anesthesia. Possible complications: intramuscular injection of a local anesthetic solution with the wrong position of the needle, as well as rapid resorption of the anesthetic into the blood when a concentrated solution is injected. Anesthesia of the sciatic nerve from the anterior approach Topography. The sciatic nerve originates from the sacral plexus (L4-S3). It is formed in the small pelvis and leaves the pelvic cavity through the piriform opening along with the artery. Medial to it, the posterior cutaneous nerve passes, as well as the neurovascular bundle, consisting of the internal pudendal artery, vein, and pudendal nerve. In the gluteal region, the sciatic nerve lies under the gluteus maximus, behind the gemellis, obturator internus, and quadratus femoris muscles. On the latter, it is located approximately at an equal distance from the ischial tuberosity and the greater trochanter of the femur. As a rule, in the upper part of the popliteal fossa, the nerve divides into terminal branches: the tibial and common peroneal nerves. The tibial nerve passes through the middle of the popliteal fossa, located lateral and superficial to the popliteal vein and artery, and, together with the vessels, enters the ankle-popliteal canal. The tibial nerve innervates the posterior muscle group of the lower leg, all the muscles of the sole of the foot, the skin of the posterior surface of the lower leg, heel and lateral edge of the foot. Branches from the sciatic nerve to the hip joint. In the region of the gluteal fold, it lies superficially under the broad fascia of the thigh outward from the long head of the biceps femoris. The common peroneal nerve runs along the lateral side of the popliteal fossa, around the head of the fibula. From it there are branches to the knee joint and the skin of the lateral surface of the lower leg, it also innervates the muscles of the lower leg, the back muscles of the foot and the skin of the rear of the toes. Anesthesia of the sciatic nerve is performed from the anterior approach. Landmarks: anterior superior iliac axis and the most protruding part of the greater trochanter of the femur. Anesthesia technique. The patient lies on his back. The anterior superior iliac spine and the most protruding point of the greater trochanter of the femur are connected by a straight line, and a perpendicular to the anterior surface of the thigh is restored from the last point. The length of the perpendicular is equal to the distance between the anterior superior iliac spine and the greater trochanter of the femur. The end of this perpendicular is the projection point. In the physiological position of the lower limb, after treating the skin with a disinfectant solution and creating a "lemon peel", a 12-15 cm long needle is directed vertically down until it rests against the lesser trochanter of the femur. After the needle slips off the lesser trochanter, without changing the main direction, the needle is carried even deeper - 4-5 cm until paresthesia occurs. If it is not possible to achieve paresthesia, the needle is returned to the bone and, turning the limb inward by 7-10 °, the needle is advanced again until paresthesia appears in the patient. Anesthesia of the sciatic nerve from the posterior approach Anesthesia technique. The patient lies on a healthy side, the anesthetized limb is bent at the hip and knee joints at an angle of 45-60°. From the most protruding part of the greater trochanter of the femur, a straight line is drawn to the posterior superior iliac spine, from the middle of which a perpendicular 4-5 cm long is lowered in the caudal direction. The end of the perpendicular serves as a projection point for the sciatic nerve. The needle is injected at an angle of 90° to the frontal plane of the patient's body and advanced until paresthesia or contact with the bone is obtained. If necessary, the needle is pulled up and inserted approximately 0,5 cm lateral or medial to the original injection. Getting paresthesia is a must. Enter 20-25 ml of a 2% solution of lidocaine (trimecaine). The technique of anesthesia will not change if the patient lies on his stomach. Anesthesia in the popliteal fossa For surgical interventions on the lower leg and foot, it is sufficient to anesthetize the tibial and peroneal nerves in the popliteal fossa. The position of the patient - on a healthy side or on the stomach. Landmarks: tendons of the biceps, semimembranosus and semitendinosus muscles of the thigh, patella, calf muscle. Topography. The popliteal fossa contains fiber, blood vessels, nerves and lymph nodes. The upper outer border is formed by the tendon of the biceps femoris muscle, the upper inner border is formed by the tendons of the semimembranosus and semitendinosus muscles, the lateral head of the gastrocnemius muscle is below and outside, and the lower internal is the medial head of the gastrocnemius muscle. The projection of the upper angle of the popliteal fossa mostly corresponds to the upper edge of the patella in the upper corner of the rhomboid fossa of the sciatic nerve and is divided into the tibial and common peroneal nerves. The latter from the upper edge goes to the lateral edge of the gastrocnemius and wraps around the neck of the head of the fibula semi-spirally. In the popliteal fossa, the tibial nerve passes most superficially along the midline, a vein lies deeper and medially from it, and even deeper and medially, closer to the bone, is the popliteal artery. Anesthesia technique. The upper corner of the rhomboid fossa is at the level of the upper edge of the patella. The injection point lies 1-1,5 cm below the upper angle on the bisector lowered from this angle, formed from the outside by the tendon of the biceps femoris, from the inside by the tendons of the semimembranosus and semitendinosus muscles of the thigh. The direction of movement of the needle is strictly vertical with the patient in the prone position until paresthesia of the tibial nerve is obtained. In the absence of paresthesia, the needle is directed fan-shaped, pulling it up each time to the level of subcutaneous tissue. After an aspiration test, 5-10 ml of a 2% solution of trimecaine is injected. To block the common peroneal nerve from the same point, the needle is directed laterally at an angle of 30-45° to the frontal plane. After receiving paresthesia, 5-10 ml of a 2% solution of trimecaine is injected. There are no complications or contraindications. Complications of regional anesthesia During RA, complications occur quite rarely, and they can be divided into two groups. 1. Specific, which are more related to the technical errors of the methodology: 1) erroneous injection of a local anesthetic into the spinal canal or epidural space, intravenously or intra-arterially (with anesthesia of the cervical plexus, anesthesia of the lumbar plexus with inguinal access); 2) puncture of cavities and organs (pleural cavity, lung); 3) the formation of a hematoma with a neglect of puncture and damage to a large vessel; hematoma compresses the surrounding tissue or neurovascular bundle; 4) prolonged and severe hypotension that occurs with rapid resorption of the local anesthetic; 5) trauma to the nerve plexus or conductor with the end of the injection needle during rough manipulation; 6) the absence of an analgesic effect after plexus or conduction anesthesia. 2. Nonspecific, manifested mainly by general and local reactions of the body to the action of a local anesthetic in the form of toxic and allergic reactions. Non-specific complications may develop depending on the time, dose and site of administration of the anesthetic. In this case, lesions of the central nervous or cardiovascular systems predominate. Complications from the CNS can be mild (limited only to central excitation) or severe, which is manifested by CNS inhibition with possible total paralysis. The nature of non-specific complications: 1) an overdose of anesthetic with the simultaneous administration of a large dose (more than 1 g) of trimecaine or lidocaine intraarterially or intravenously. With intravascular injection of a local anesthetic, toxic reactions appear immediately, and with overdoses of the drug - after 10-15 minutes. Severe toxic reactions with excessive dosages of local anesthetics are extremely rare. Much more often (up to death) they are observed with intravascular administration of a highly concentrated solution of local anesthetic; 2) allergic reactions to the introduction of a local anesthetic, characterized by a symptom complex of disorders of the central nervous and cardiovascular systems: anxiety, impaired consciousness, speech disorders, muscle twitches, convulsions, deep breathing, heart rhythm disturbance, QR expansion, lowering blood pressure, tachycardia, apnea , asystole; 3) infectious complications due to poor-quality processing of the surgical field at the site of anesthesia, insufficient sterilization of syringes, needles and solutions, as well as in the presence of a local skin infection (pyoderma) in the area of the intended manipulation; 4) the development of hypotension with plexus and conduction anesthesia is much less common than with epidural and spinal anesthesia, but this does not reduce the importance of correcting hypovolemia; 5) with the introduction of concentrated solutions of local anesthetics in a small percentage of cases, the development of bradycardia is noted, which is well stopped by atropine sulfate. Cases of ventricular fibrillation and cardiac arrest are extremely rare. Contraindications to Contraindications are as follows. 1. Hysteria. 2. Tendency to aggravate. 3. Neurological complications: hemiplegia and hemiparesis of the proposed area of anesthesia, diseases of the spinal cord, multiple sclerosis, diseases of the peripheral nerves. 4. Infection of the skin in the area of the needle injection point. 5. Septicemia. 6. Tendency to bleeding and severe hypovolemia after massive blood loss. 7. Pernicious anemia (relative contraindication). 7. Epidural anesthesia Its essence lies in the blockade of the spinal nerves and their roots with an anesthetic introduced into the epidural space. The technique of conducting and the zone of anesthesia are indicated in lecture No. 2. Doses and duration of analgesia 1. Lidocaine is injected in a volume of 4 ml (2%), the duration of anesthesia is 4 hours. 2. Marcaine is administered at a dose of 6-10 ml (0,5%), the duration of anesthesia is 6-8 hours. 3. Naropin is administered at a dose of 4-6 ml (1%), the duration of anesthesia is 4-6 hours. With this type of anesthesia, hemodynamics suffers, there is a sharp decrease in blood pressure. Therefore, when using this type of anesthesia, constant monitoring of the patient's pressure and, if necessary, its correction is necessary. 8. Lumbar anesthesia This type of anesthesia is achieved by injecting an anesthetic into the spinal canal. The technique of lumbar puncture is described in lecture No. 2. Features are that the level of puncture is higher (T12-L1, L1-L2) and after removing the mandrin from the needle, an anesthetic is immediately injected. The volume of anesthetic is 3-5 ml. Naropin is administered in 0,5% concentration, lidocaine - in 2%, marcaine - in 0,5%. The duration of anesthesia is 4-6 hours. This type of anesthesia also causes severe hypotension. With a high level of anesthesia (T12-L1) may cause respiratory problems. The most common complication is headache. 9. Cervical vagosympathetic blockade according to A. V. Vishnevsky The patient is placed on the table on his back so that the hand on the side of the proposed blockade hangs over the edge of the table. The sternocleidomastoid muscle should be relaxed. A roller is placed under the shoulder girdle, the head is retracted in the opposite direction; in this position, the anatomical contours of the neck are well outlined. The field is treated with lubrication with alcohol and iodine. The doctor stands on the side of the blockade of the same name. The injection site is the angle formed by the intersection of the posterior edge of the sternocleidomastoid muscle with a vein. The index finger of the left hand is placed at the posterior edge of the sternocleidomastoid muscle, above the place where it crosses with the external jugular vein. Strongly pressing a finger on this place, they try to move the neurovascular bundle to the midline. In this case, the finger easily feels the anterior surface of the cervical vertebrae. The injection of the needle and its advancement should be done slowly, upward and inward, all the while focusing on the anterior surface of the spine. The needle moves along the 2% novocaine solution sent in small portions (3-0,25 cm), which ensures the safety of the injection. The syringe during the injection is repeatedly removed from the needle for the purpose of control (blood). Having brought the needle to the vertebra, they feel how it rests against it. Then the pressure on the needle is weakened, due to which it moves away by 1-2 mm, after which 40 to 60 ml of a solution is injected, which, spreading in a creeping infiltrate along the prevertebral aponeurosis, covers the vagus, sympathetic, and often phrenic nerves, interrupting ( blocking) transmission of irritations from the pleuropulmonary region. The effectiveness of vagosympathetic blockade is evidenced by the appearance of Horner's symptom (narrowing of the pupil, narrowing of the palpebral fissure and retraction of the eyeball). The respiratory rhythm and pulse become less frequent, shortness of breath and cyanosis decrease, and the general condition improves. To avoid getting the needle into the vessels of the neck, the sternocleidomastoid muscle with the underlying neurovascular bundle should be moved strongly enough with the index finger to the midline. It is important to direct the needle up and inward, since moving it in a horizontal direction threatens to introduce a solution under the prevertebral aponeurosis and subsequent complication in the form of a pain syndrome that does not stop during the day. Lecture No. 14. Assessment of the severity of the patient and monitoring Assessment of the somatic condition of the patient. 1 point - patients whose disease is localized and, as a rule, does not cause systemic disorders, i.e. practically healthy people. 2 points - this includes patients with unexpressed disorders that moderately disrupt the vital activity of the body without pronounced shifts in homeostasis. 3 points - patients with vital systemic disorders that significantly disrupt the functioning of the body, but do not lead to disability. 4 points - patients with severe systemic disorders that pose a high danger to human life and lead to disability. 5 points - patients whose condition is considered critical, the risk of death within 24 hours is high. The volume and nature of the surgical intervention: 1 point - small operations for: removal of superficially located and localized tumors, opening of small abscesses, amputation of fingers and toes, ligation and removal of hemorrhoids, uncomplicated appendectomy and herniotomy. 2 points - operations of moderate severity: removal of superficially located malignant tumors requiring extended intervention; opening of abscesses localized in cavities; amputation and disarticulation of the upper and lower extremities; operations on peripheral vessels; complicated appendectomy and herniotomy requiring extensive intervention; trial thoracotomy and laparotomy; others similar in complexity and scope of intervention. 3 points - extensive surgical interventions: radical operations on the abdominal organs (except those listed above); radical operations on the organs of the chest cavity; extended limb amputations, such as transiliosacral amputation; brain surgery. 4 points - operations on the heart, large vessels and other complex interventions performed under special conditions (artificial circulation, hypothermia, etc.). Emergency surgical interventions are evaluated, as well as planned ones, according to the physical condition and volume of the operation, but they are included in a separate group or denoted in addition to the figure by the index "C". Note Operational and anesthetic risk is designated as follows: the numerator indicates the severity of the initial condition in points, the denominator indicates the volume of the operation, also in points. Classification of the objective status of the patient, developed by the American Society of Anesthesiologists (ASA). 1. There are no systemic disorders. 2. Mild systemic disorders without functional impairment. 3. Moderate and severe systemic diseases with dysfunction. 4. Severe systemic disease that constantly poses a threat to life and leads to failure of functions. 5. Terminal state, high risk of death during the day, regardless of the operation. 6. Brain death, organ donation for transplantation. If the intervention is carried out on an emergency basis, the assessment of the state is supplemented by the letter "E". The assessment of the patient's condition and the possible lethality of each case are shown in Table 7. Table 7 Assessment of the patient's condition and perioperative mortality
Monitoring (Table 7) is the control of functions and processes, the identification of their dangerous deviations in order to prevent complications, in particular, during anesthesia and intensive care. Monitoring is carried out to control: 1) for the functions of the patient (electrocardiography, pulse oximetry, capnography, etc.); 2) therapeutic actions (control of the neuromuscular block); 3) environment (gas composition of the inhaled mixture); 4) operation of technical means (ventilator, etc.). Monitoring of functions by degree of complexity can be carried out by: 1) continuous monitoring of parameters; 2) control with signaling when a parameter fails beyond the established limits; 3) continuous monitoring with notification when the parameter goes beyond the established limits, and additionally a hint for the solution; 4) the same with the implementation of measures to normalize the function. The relevance of monitoring is due to: 1) the ever-increasing complexity and duration of surgical interventions; 2) an increase in the severity of functional disorders in patients; 3) the complication of technical means used in critical medicine. Significance of monitoring: 1) timely diagnosis of disorders and prevention of severe complications, including cardiac and respiratory arrest; 2) more adequate tactics of intensive care and high efficiency of treatment. Monitoring indications: 1) minimal monitoring - always mandatory during anesthesia and intensive care; 2) in-depth monitoring (non-invasive and invasive methods) in case of significant violations of the body's functions, especially when a patient develops multiple organ failure; 3) preventive monitoring - at the risk of developing a critical condition. Monitoring standards. In 1978, the Dutch Board of Health introduced the first operating room monitoring standard, which listed the equipment needed for monitoring. In 1985, at the suggestion of insurance companies, the Harvard monitoring standard for anesthesiology was proposed, which provides parameters for monitoring patients during anesthesia during surgery and the mode of such monitoring. Its introduction into practice reduced the risk of anesthetic complications and made it safer for the patient. In Russia, in 1997, the MORF system outlined standards for minimal monitoring of anesthesia, resuscitation and intensive care (No. 161/DM-2 of February 24, 1997 “On measures to ensure the safety of patients during anesthesia, resuscitation and intensive care "). The data is shown in table 8, table 9. Table 8 Anesthesia
Table 9 Conducting resuscitation and intensive care
To conduct effective intensive care, it is necessary to monitor the cardiovascular, respiratory and nervous systems, the functions of the liver, kidneys, gastrointestinal tract, hematopoiesis, hemostasis, as well as energy, water-electrolyte, acid-base and onco-osmotic balance. Equally important is the intensive monitoring of ongoing therapeutic interventions and their results. An important role is played by monitoring the external and internal microbiological status, as well as the use of prognostic criteria and outcome assessment. First of all, it is necessary to use a clinical assessment of the patient's status and non-invasive monitoring methods. Clinical monitoring, i.e. the observation of clinical signs and symptoms, qualitative data, is no less important than quantitative indicators obtained using sophisticated technology. System function monitoring Monitoring of the circulatory system Circulation monitoring provides timely detection of cardiac arrhythmias and myocardial ischemia through the use of electrocardiography. Cardiac arrhythmias can be identified by the P wave and the ORS complex on the electrocardiomonitor in leads V1 and V2 of the standard lead from the limbs or their modifications. Myocardial ischemia can be identified by the resulting depression of the ST segment on the ECG: 1) in the chest lead V5, as well as on one of its modifications - ischemia of the septum of the left side wall; 2) standard 2 lead from the extremities - ischemia of the basal zone of the myocardium in the basin of the right coronary artery. Downsloping ST depression (elevation) is an indicator of stress-induced ischemia. Monitoring of hemodynamics is carried out by: 1) blood pressure measurements; 2) CVP measurements in combination with volumetric stress tests (this is information about vascular filling); 3) determination of pulmonary artery pressure and wedge pressure (PWP) using a floating pulmonary artery catheter is a more accurate method for assessing intravascular volume than CVP, and can also serve as a measure of left ventricular preload; 4) determination of cardiac output using a thermodilution technique, the Fick method (CO = VCO2 /CaCO2), various modifications of the Doppler technique (esophageal Doppler echocardiography), ultrasound of the heart. Breath monitoring Respiratory monitoring is carried out according to clinical symptoms and data of capnography, pulse oximetry, volume spirometry and periodic blood gas examination. During mechanical ventilation, the pressure in the "ventilator - patient" system and the oxygen concentration in the inhaled mixture (FiO2). Clinical signs of respiratory failure: frequent (more than 24-30 per minute for adults) shallow breathing, participation in breathing of additional muscles (sternocleidomastoid, abdominal and others, which is manifested by retraction of the intercostal spaces, swelling of the wings of the nose, forced half-sitting position), sweating, cyanosis, a change in heart rate (first increase, and then there may be arrhythmia) and blood pressure (increase, and with severe hypoxia - decrease), a change in consciousness from euphoria to coma. Capnography allows timely detection of ventilation disorders: hypoventilation (increased CO2 in the final exhaled air - FetCO2 > 6,4%), hyperventilation (FetCO2 < 4,9%), uneven ventilation (angle of inclination of the alveolar capnogram plateau - 5°). During mechanical ventilation, if there is no capnograph, the volume of ventilation is controlled by the minute volume of respiration (Vist.), measured using a volumetric spirometer, which is installed in the expiratory path. In addition, the minute inspiratory volume (Vappar.) is monitored, which is necessary to calculate the oxygen concentration in the inhaled gas mixture and determine the tightness of this system - "ventilator-patient". The tightness control is also achieved by the pressure in the "device-sick" system, measured by means of a monovacuometer. Pulse oximetry allows timely detection of impaired oxygenation in the lungs, hypoxemia (SaO2 < 94%. In addition, based on the nature of the plethysmogram, one can judge the state of microcirculation and cardiac output. An additional study of blood gases helps to assess the degree of impaired gas exchange in the lungs (by the magnitude of the alveolo-arterial gradient of oxygen tension. Some monitors can provide an assessment of the biomechanics of breathing during mechanical ventilation based on the compliance of the lungs and chest (C, normally 60-100 ml) and resistance (resistance) of the respiratory tract (R, normally 2-3 cm). Monitoring of neurological functions Monitoring of neurological functions is carried out by assessing consciousness on the Glasgow scale (based on the eye opening reaction, motor and verbal responses to an increasing stimulus: 15 points - normal, 3 points - brain death). In addition, intracranial pressure and cerebral blood flow are determined (for example, using a transcranial Doppler monitor). Renal function is most commonly monitored by measuring hourly urine output, which is normally > 0,5 ml/kg/h. Lecture number 15. Artificial lung ventilation Artificial lung ventilation (ALV) provides gas exchange between the surrounding air (or a certain mixture of gases) and the alveoli of the lungs, is used as a means of resuscitation in the event of a sudden cessation of breathing, as a component of anesthesia and as a means of intensive care for acute respiratory failure, as well as some diseases of the nervous and muscular systems. Modern methods of artificial lung ventilation (ALV) can be divided into simple and hardware. A simple method of mechanical ventilation is usually used in emergency situations (apnea, with an abnormal rhythm, agonal breathing, with increasing hypoxemia and (or) hypercapnia, and severe metabolic disorders). The expiratory methods of IVL (artificial respiration) from mouth to mouth and from mouth to nose are simple. Hardware methods are used if necessary for long-term mechanical ventilation (from one hour to several months and even years). The Phase-50 respirator has great potential. For pediatric practice, the apparatus "Vita-1" is produced. The respirator is connected to the patient's airways through an endotracheal tube or tracheostomy cannula. Hardware ventilation is carried out in the normal frequency mode, which ranges from 12 to 20 cycles per 1 min. In practice, there are mechanical ventilation in high-frequency mode (more than 60 cycles per 1 min), in which the tidal volume decreases markedly (up to 150 ml or less), positive pressure in the lungs at the end of inspiration decreases, as well as intrathoracic pressure, and blood flow to the heart improves. Also, in high-frequency mode, the patient's adaptation to the respirator is facilitated. There are three methods of high-frequency ventilation: volumetric, oscillatory and jet. Volume is usually carried out with a respiratory rate of 80-100 per 1 min, oscillatory IVL - 600-3600 per 1 min, which ensures the vibration of a continuous or intermittent gas flow. The most widespread jet high-frequency ventilation with a respiratory rate of 100-300 per minute, in which a jet of oxygen at a pressure of 1-2 atm is blown into the airways through a needle or catheter with a diameter of 2-4 mm. Jet ventilation is carried out through an endotracheal tube or tracheostomy (at the same time, atmospheric air is sucked into the respiratory tract) and through a catheter that is inserted into the trachea through the nasal passage or percutaneously (puncture). The latter is important in situations where there are no conditions for tracheal intubation. Artificial ventilation of the lungs can be carried out in automatic mode, but this is acceptable in cases where the patient's spontaneous breathing is completely absent or suppressed by pharmacological drugs (muscle relaxants). Assisted ventilation is also carried out, but in this case, the patient's independent breathing is preserved. Gas is supplied after the patient makes a weak attempt to inhale, or the patient is synchronized to an individually selected mode of operation of the apparatus. There is also an Intermittent Mandatory Ventilation (PMV) mode, which is applied during the gradual transition from mechanical ventilation to spontaneous breathing. In this case, the patient breathes on his own, but additionally, a continuous flow of the gas mixture is supplied to the airways. Against this background, with a specified frequency (from 10 to 1 time per minute), the device performs an artificial breath, coinciding (synchronized PVL) or not coinciding (non-synchronized PVL) with the patient's independent inspiration. The gradual reduction of artificial breaths allows you to prepare the patient for spontaneous breathing. Breathing circuits are shown in Table 10. Table 10 Breathing circuits
Manual ventilation with a bag or mask is readily available and is often sufficient to adequately inflate the lungs. Its success, as a rule, is determined by the correct selection of the size of the mask and the experience of the operator, and not by the severity of the lung pathology. Показания 1. Resuscitation and preparation of the patient in a short period of time for subsequent intubation. 2. Periodic ventilation with a bag and mask to prevent post-extubation atelectasis. 3. Restrictions on ventilation with a bag and a mask. Equipment A conventional breathing bag and a mask with an installed pressure gauge or a self-inflating breathing bag with an oxygen chamber are used. Techniques for conducting 1. It is necessary to place the mask tightly on the patient's face, giving the patient's head a median position with the chin fixed with a finger. The mask should not lie on the eyes. 2. Respiratory rate - usually 30-50 per 1 min. 3. Inspiratory pressure - usually 20-30 cm of water. Art. 4. Greater pressure (30-60 cm of water column) is acceptable during primary resuscitation in the labor activity of a woman. Efficiency mark 1. Return of heart rate to normal numbers and the disappearance of central cyanosis. 2. Excursion of the chest should be good, breathing is carried out equally well on both sides. 3. The study of the gas composition of the blood is usually required and carried out during prolonged resuscitation. Complications 1. Pneumothorax. 2. Bloating. 3. Hypoventilation syndrome or episodes of apnea. 4. Irritation of the skin of the face. 5. Retinal detachment (when applying a mask to the eyes and creating a long-term high peak pressure). 6. Mask and bag ventilation may worsen the patient's condition if he actively resists the procedure. Hardware IVL Показания 1. Apnea. 2. Coma in the acute period, even without signs of respiratory failure. 3. Seizures not controlled by standard anticonvulsant therapy. 4. Shock of any etiology. 5. Increase in the dynamics of the syndrome of CNS depression in hyperventilation syndrome. 6. With a birth spinal injury in newborns - the appearance of forced breathing and crepitating widespread wheezing against the background of shortness of breath. 7. RO2 capillary blood less than 50 mm Hg. Art. during spontaneous breathing with a mixture with FiO2 0,6 or more. 8. RSO2 capillary blood more than 60 mm Hg. Art. or less than 35 mm Hg. Art. with spontaneous breathing. Equipment: "PHASE-5", "BP-2001", "Infant-Star 100 or 200", "Sechrist 100 or 200", "Babylog 1", "Stephan", etc. Principles of treatment 1. Oxygenation in stiff lungs can be achieved by increasing the inspired oxygen concentration, increasing the inspiratory pressure, increasing the PEEP, prolonging the inspiratory time, increasing the plateau pressure. 2. Ventilation (removal of CO2) can be enhanced by an increase in tidal volume, an increase in frequency, and a prolongation of expiratory time. 3. The selection of ventilation parameters (frequency, inspiratory pressure, inspiratory plateau, inspiratory-expiratory ratio, PEEP) will vary depending on the nature of the underlying disease and the patient's response to therapy. Purposes of IVL 1. Oxygen: reach pO2 50-100 mmHg Art. 2. Hold pCO2 within 35-45 mm Hg. Art. 3. Exceptions: in some situations, pO indicators2 and pCO2 may differ from the above: 1) in chronic pulmonary pathology, higher pCO values2 portable; 2) with severe heart defects, smaller pO numbers are tolerated2; 3) depending on the therapeutic approach in the case of pulmonary hypertension, larger or smaller pCO values are tolerated2. 4. Indications and ventilation parameters should always be documented. Techniques for conducting 1. Initial parameters of IVL: inspiratory pressure 20-24 cm of water. Art.; PEER from 4-6 cm of water. Art.; respiratory rate 16-24 per 1 min, inhalation time 0,4-0,6 s, DO from 6 to 10 l / min, MOV (minute ventilation volume) 450-600 ml / min. 2. Synchronization with a respirator. As a rule, patients are synchronous with the respirator. But excitement can impair synchronization, in such cases, drug therapy (morphine, promedol, sodium oxybutyrate, muscle relaxants) may be required. Examination 1. An important component of the survey are repeated blood gas tests. 2. Physical examination. Control of the adequacy of the IVL. When performing emergency ventilation with a simple method, it is enough to observe the color of the skin and movements of the patient's chest. The chest wall should expand with each inhalation and fall with each exhalation, but if the epigastric region rises, then the blown air enters the esophagus and stomach. The reason is often the wrong position of the head of the patient. When conducting long-term mechanical ventilation, it is necessary to judge its adequacy. If the patient's spontaneous breathing is not suppressed by pharmacological preparations, then one of the main signs of the adequacy of the IVL performed is the good adaptation of the patient to the respirator. In the presence of a clear consciousness, the patient should not have a feeling of lack of air, discomfort. Breath sounds in the lungs should be the same on both sides, and the skin should have a normal color. Complications 1. The most common complications of mechanical ventilation are: rupture of the alveoli with the development of interstitial emphysema, pneumothorax and pneumomediastinitis. 2. Other complications can be: bacterial contamination and infection, obturation of the endotracheal tube or extubation, one-lung intubation, pneumopericarditis with cardiac tamponade, decreased venous return and decreased cardiac output, chronicity of the process in the lungs, stenosis and obstruction of the trachea. Against the background of mechanical ventilation, it is possible to use a number of analgesics, which should provide a sufficient level and depth of anesthesia in doses, the introduction of which under conditions of spontaneous breathing would be accompanied by hypoxemia. By maintaining a good supply of oxygen to the blood, mechanical ventilation contributes to the fact that the body copes with the surgical injury. In many operations on the organs of the chest (lungs, esophagus), separate bronchial intubation is used, which makes it possible to turn off one lung from ventilation during surgical interventions in order to facilitate the work of the surgeon. Such intubation also prevents the contents from the operated lung from flowing into the healthy lung. During operations on the larynx and respiratory tract, transcatheter jet high-frequency ventilation is used, which facilitates the examination of the surgical field and allows maintaining adequate gas exchange with the trachea and bronchi opened. Under conditions of general anesthesia and muscle relaxation, the patient is not able to respond to the resulting hypoxia and hypoventilation, therefore, it is important to control the blood gas content (continuous monitoring of oxygen partial pressure and carbon dioxide partial pressure) by percutaneous means using special sensors. In case of clinical death or agony, mechanical ventilation is a mandatory component of resuscitation. It is possible to stop carrying out mechanical ventilation only after the consciousness is completely restored and spontaneous breathing is complete. In the complex of intensive care, mechanical ventilation is the most effective method for the treatment of acute respiratory failure. It is carried out through a tube that is inserted into the trachea through the lower nasal passage or tracheostomy. Of particular importance is the care of the respiratory tract, their adequate drainage. Auxiliary mechanical ventilation is used in sessions for 30-40 minutes to treat patients with chronic respiratory failure. IVL is used in patients in a state of coma (trauma, brain surgery), as well as with peripheral damage to the respiratory muscles (polyradiculoneuritis, spinal cord injury, amyotrophic lateral sclerosis). ALV is also widely used in the treatment of patients with chest trauma, various poisonings, cerebrovascular accidents, tetanus, and botulism. Lecture No. 16. Infusion therapy Infusion therapy is a drip injection or infusion intravenously or under the skin of drugs and biological fluids in order to normalize the water-electrolyte, acid-base balance of the body, as well as for forced diuresis (in combination with diuretics). Indications for infusion therapy: all types of shock, blood loss, hypovolemia, loss of fluid, electrolytes and proteins as a result of indomitable vomiting, intense diarrhea, refusal to take fluids, burns, kidney disease; violations of the content of basic ions (sodium, potassium, chlorine, etc.), acidosis, alkalosis and poisoning. The main signs of dehydration of the body: retraction of the eyeballs into the orbits, dull cornea, dry, inelastic skin, characteristic palpitations, oliguria, urine becomes concentrated and dark yellow, the general condition is depressed. Contraindications to infusion therapy are acute cardiovascular failure, pulmonary edema and anuria. Crystalloid solutions are able to compensate for the deficiency of water and electrolytes. Apply 0,85% sodium chloride solution, Ringer and Ringer-Locke solutions, 5% sodium chloride solution, 5-40% glucose solutions and other solutions. They are administered intravenously and subcutaneously, by stream (with severe dehydration) and drip, in a volume of 10-50 ml/kg or more. These solutions do not cause complications, except for overdose. The goals of infusion therapy are: restoration of BCC, elimination of hypovolemia, ensuring adequate cardiac output, maintaining and restoring normal plasma osmolarity, ensuring adequate microcirculation, preventing aggregation of blood cells, normalizing the oxygen transport function of blood. Colloidal solutions are solutions of macromolecular substances. They contribute to the retention of fluid in the vascular bed. Hemodez, polyglucin, reopoliglyukin, reogluman are used. With their introduction, complications are possible, which manifest themselves in the form of an allergic or pyrogenic reaction. Routes of administration - intravenously, less often subcutaneously and drip. The daily dose does not exceed 30-40 ml/kg. They have a detoxifying quality. As a source of parenteral nutrition, they are used in case of prolonged refusal to eat or inability to feed by mouth. Blood and casein hydrolysins are used (alvezin-neo, polyamine, lipofundin, etc.). They contain amino acids, lipids and glucose. Sometimes there is an allergic reaction to the introduction. Rate and volume of infusion. All infusions in terms of volumetric infusion rate can be divided into two categories: requiring and not requiring rapid correction of the BCC deficiency. The main problem may be patients who need rapid elimination of hypovolemia. i.e., the rate of infusion and its volume must ensure the performance of the heart in order to properly supply regional perfusion of organs and tissues without significant centralization of blood circulation. In patients with an initially healthy heart, three clinical landmarks are most informative: mean BP > 60 mm Hg. Art.; central venous pressure - CVP> 2 cm of water. Art.; diuresis 50 ml/h. In doubtful cases, a test with a load in volume is carried out: 15-20 ml of a crystalloid solution is poured over 400-500 minutes and the dynamics of CVP and diuresis are observed. A significant rise in CVP without an increase in diuresis may indicate heart failure, which suggests the need for more complex and informative methods for assessing hemodynamics. Keeping both values low suggests hypovolemia, then a high infusion rate is maintained with repeated step-by-step assessment. An increase in diuresis indicates prerenal oliguria (hypoperfusion of the kidneys of hypovolemic origin). Infusion therapy in patients with circulatory insufficiency requires a clear knowledge of hemodynamics, large and special monitoring monitoring. Dextrans are colloidal plasma substitutes, which makes them highly effective in the rapid recovery of BCC. Dextrans have specific protective properties against ischemic diseases and reperfusion, the risk of which is always present during major surgical interventions. The negative aspects of dextrans include the risk of bleeding due to platelet disaggregation (especially characteristic of rheopolyglucin), when it becomes necessary to use significant doses of the drug (> 20 ml / kg), and a temporary change in the antigenic properties of the blood. Dextrans are dangerous because of their ability to cause a "burn" of the epithelium of the tubules of the kidneys and therefore are contraindicated in renal ischemia and renal failure. They often cause anaphylactic reactions, which can be quite severe. Of particular interest is a solution of human albumin, as it is a natural colloid of a plasma substitute. In many critical conditions accompanied by damage to the endothelium (primarily in all types of systemic inflammatory diseases), albumin is able to pass into the intercellular space of the extravascular bed, attracting water and worsening interstitial tissue edema, primarily the lungs. Fresh frozen plasma is a product taken from a single donor. FFP is separated from whole blood and frozen immediately within 6 hours of blood sampling. Stored at 30°C in plastic bags for 1 year. Given the lability of clotting factors, FFP should be infused within the first 2 hours after rapid thawing at 37°C. Transfusion of fresh frozen plasma (FFP) gives a high risk of contracting dangerous infections, such as HIV, hepatitis B and C, etc. The frequency of anaphylactic and pyrogenic reactions during transfusion of FFP is very high, so compatibility according to the ABO system should be taken into account. And for young women, Rh-compatibility must be considered. Currently, the only absolute indication for the use of FFP is the prevention and treatment of coagulopathic bleeding. FFP performs two important functions at once - hemostatic and maintaining oncotic pressure. FFP is also transfused with hypocoagulation, with an overdose of indirect anticoagulants, with therapeutic plasmapheresis, with acute DIC, and with hereditary diseases associated with a deficiency of blood coagulation factors. Indicators of adequate therapy are a clear consciousness of the patient, warm skin, stable hemodynamics, the absence of severe tachycardia and shortness of breath, sufficient diuresis - within 30-40 ml/h. 1. Blood transfusion Complications of blood transfusion: post-transfusion disorders of the blood coagulation system, severe pyrogenic reactions with the presence of hyperthermic syndrome and cardiovascular decompensation, anaphylactic reactions, erythrocyte hemolysis, acute renal failure, etc. The basis of most complications is the reaction of rejection by the body of foreign tissue. There are no indications for transfusion of canned whole blood, because the risk of post-transfusion reactions and complications is significant, but the most dangerous is the high risk of infection of the recipient. In case of acute blood loss during surgical intervention and adequate replenishment of the BCC deficiency, even a sharp decrease in hemoglobin and hematocrit does not threaten the life of the patient, since oxygen consumption under anesthesia is significantly reduced, additional oxygenation is acceptable, hemodilution helps prevent the occurrence of microthrombosis and mobilization of erythrocytes from the depot, increase blood flow velocity and etc. The "reserves" of erythrocytes that a person has by nature significantly exceed the real needs, especially in a state of rest, in which the patient is at this time. Recommendations for the appointment of transfusion of donor blood and erythrocytes during surgery. 1. Transfusion of erythrocyte mass is carried out after the restoration of the BCC. 2. In the presence of severe concomitant pathology, which can lead to death (for example, severe anemia is poorly tolerated in severe coronary heart disease). 3. In the presence of the following indicators of the patient's red blood: 70-80 g / l for hemoglobin and 25% for hematocrit, and the number of red blood cells is 2,5 million. Indications for blood transfusion are: bleeding and correction of hemostasis. Types of erythrocytes: whole blood, erythrocyte mass, EMOLT (erythrocyte mass separated from leukocytes, platelets with saline). Blood is administered intravenously by drip, using a disposable system at a rate of 60-100 drops per minute, in a volume of 30-50 ml/kg. Before blood transfusion, it is necessary to determine the blood type and Rh factor of the recipient and donor, conduct a test for their compatibility, and a biological test for compatibility is performed at the patient's bedside. When an anaphylactic reaction occurs, the transfusion is stopped and measures to eliminate shock begin. Standard platelet concentrate is a suspension of twice centrifuged platelets. The minimum platelet count is 0,5 x 1012 per liter, leukocytes - 0,2 x 109 per liter. Hemostatic characteristics and survival are most pronounced in the next 12-24 hours of preparation, but the drug can be used within 3-5 days from the moment of blood sampling. Platelet concentrate is used for thrombocytopenia (leukemia, bone marrow aplasia), thrombopathy with hemorrhagic syndrome. 2. Parenteral nutrition In severe diseases accompanied by severe disturbances of homeostasis, it is necessary to provide the body with energy and plastic material. Therefore, when nutrition through the mouth is impaired or completely impossible for some reason, it is necessary to transfer the patient to parenteral nutrition. In critical conditions of various etiologies, the most significant changes occur in protein metabolism - intensive proteolysis is observed, especially in striated muscles. Depending on the severity of the ongoing process, body proteins are catabolized in the amount of 75-150 g per day (daily protein losses are shown in Table 11). This leads to a deficiency of essential amino acids, which are used as an energy source during gluconeogenesis, resulting in a negative nitrogen balance. Table 11 Daily protein loss in critical conditions
The loss of nitrogen leads to a decrease in body weight, since: 1 g of nitrogen \u6,25d 25 g of protein (amino acids) \uXNUMXd XNUMX g of muscle tissue. Within a day from the onset of a critical condition, without adequate therapy with the introduction of a sufficient amount of essential nutrients, its own reserves of carbohydrates are exhausted, and the body receives energy from proteins and fats. In this regard, not only quantitative, but also qualitative changes in metabolic processes are carried out. The main indications for parenteral nutrition are: 1) anomalies in the development of the gastrointestinal tract (esophageal atresia, pyloric stenosis, and others, pre- and postoperative period); 2) burns and injuries of the oral cavity and pharynx; 3) extensive body burns; 4) peritonitis; 5) paralytic ileus; 6) high intestinal fistulas; 7) indomitable vomiting; 8) coma; 9) severe diseases accompanied by increased catabolic processes and decompensated metabolic disorders (sepsis, severe forms of pneumonia); 10) atrophy and dystrophy; 11) anorexia due to neuroses. Parenteral nutrition should be carried out in conditions of compensation for volemic, water-electrolyte disorders, elimination of microcirculation disorders, hypoxemia, metabolic acidosis. The basic principle of parenteral nutrition is to provide the body with an adequate amount of energy and protein. For the purpose of parenteral nutrition, the following solutions are used. Carbohydrates: The most acceptable drug used at any age is glucose. The ratio of carbohydrates in the daily diet should be at least 50-60%. For complete utilization, it is required to maintain the rate of administration, glucose should be supplied with ingredients - insulin 1 unit per 4 g, potassium, coenzymes involved in energy utilization: pyridoxal phosphate, cocarboxylase, lipoic acid, and ATP - 0,5-1 mg / kg per day intravenously. When properly administered, highly concentrated glucose does not cause osmotic diuresis and a significant increase in blood sugar levels. For nitrogen nutrition, either high-quality protein hydrolysates (aminosol, aminone) or solutions of crystalline amino acids are used. These drugs successfully combine essential and non-essential amino acids, they are low toxic and rarely cause an allergic reaction. Doses of administered protein preparations depend on the degree of violation of protein metabolism. With compensated disorders, the dose of protein administered is 1 g/kg of body weight per day. Decompensation of protein metabolism, manifested by hypoproteinemia, a decrease in the albumin-globulin ratio, an increase in urea in daily urine, requires the introduction of increased doses of protein (3-4 g/kg per day) and anti-catabolic therapy. This includes anabolic hormones (retabolil, nerabolil - 25 mg intramuscularly 1 time in 5-7 days), building a parenteral nutrition program in the hyperalimentation mode (140-150 kcal / kg of body weight per day), protease inhibitors (kontrykal, trasylol 1000 U / kg per day for 5-7 days). For adequate assimilation of plastic material, each gram of introduced nitrogen must be provided with 200-220 kcal. Amino acid solutions should not be administered with concentrated glucose solutions, as they form toxic mixtures. Relative contraindications to the introduction of amino acids: renal and hepatic failure, shock and hypoxia. Fat emulsions containing polyunsaturated fatty acids are used to correct fat metabolism and increase the caloric content of parenteral nutrition. Fat is the most high-calorie product, however, for its utilization, it is necessary to maintain optimal doses and the rate of administration. Fat emulsions should not be administered together with concentrated polyionic glucose solutions, as well as before and after them. Contraindications for the introduction of fat emulsions: liver failure, lipemia, hypoxemia, shock conditions, thrombohemorrhagic syndrome, microcirculation disorders, cerebral edema, hemorrhagic diathesis. The required data of the main ingredients for parenteral nutrition are given in Table 12 and Table 13. Table 12 Doses, rates, calorie content of the main ingredients for parenteral nutrition
When prescribing parenteral nutrition, it is necessary to introduce optimal doses of vitamins that are involved in many metabolic processes, being coenzymes in energy utilization reactions. Table 13 Doses of vitamins (in mg per 100 kcal) required during parenteral nutrition
The program of parenteral nutrition, carried out in any mode, should be drawn up in terms of a balanced ratio of ingredients. The optimal ratio of proteins, fats, carbohydrates is 1: 1,8: 5,6. For the breakdown and inclusion of proteins, fats and carbohydrates in the process of synthesis, a certain amount of water is necessary. The ratio between the need for water and the calorie content of food is 1 ml H2O - 1 kcal (1: 1). Calculation of the need for resting energy consumption (ERP) according to Harris-Benedict: Men - EZP = 66,5 + 13,7 x weight, kg + 5 x height, cm - 6,8 x age (years). Women - EZP = 66,5 + 9,6 x weight, kg + 1,8 x height, cm - 4,7 x age (years). The EZP value, determined by the Harris-Benedict formula, averages 25 kcal/kg per day. After the calculation, the patient's physical activity factor (PFA), the metabolic activity factor (FMA) based on the clinical status, and the temperature factor (TF) are selected, with the help of which the energy requirement (E) of a particular patient will be determined. The coefficient for calculating FFA, FMA and TF are shown in Table 14. Table 14 Coefficient for calculating FFA, FMA and TF
To determine the daily PE, the EZP value is multiplied by FFA, FMA and TF. 3. Detoxification therapy In severe intoxication, active detoxification therapy is necessary, aimed at binding and removing toxins from the body. For this purpose, solutions of polyvinylpyrrolidone (neocompensan, gemodez) and gelatinol are most often used, adsorbing and neutralizing toxins, which are then excreted by the kidneys. These solutions are administered dropwise in an amount of 5-10 ml/kg of the patient's weight, adding vitamin C and potassium chloride solution in a minimum amount of 1 mmol/kg of body weight. Mafusol, which is an effective antihypoxant and antioxidant, also has a pronounced detoxifying property. In addition, it improves microcirculation and rheological properties of blood, which also contributes to the detoxification effect. With various poisonings, one of the most effective methods of detoxification is forced diuresis. Intravenous fluids for the purpose of forced diuresis are prescribed for severe degrees of poisoning and for milder ones, when the patient refuses to drink. Contraindications to forced diuresis are: acute cardiovascular failure and acute renal failure (anuria). Carrying out forced diuresis requires strict accounting of the volume and quantitative composition of the injected fluid, the timely appointment of diuretics, clear clinical and biochemical control. As the main solution for water load, it is proposed: glucose 14,5 g; sodium chloride 1,2 g; sodium bicarbonate 2,0 g; potassium chloride 2,2 g; distilled water up to 1000 ml. This solution is isotonic, contains the required amount of sodium bicarbonate, the concentration of potassium in it does not exceed the permissible one, and the ratio of the osmotic concentration of glucose and salts is 2: 1. At the initial stage of forced diuresis, it is also advisable to introduce plasma-substituting and any detoxification solutions: albumin 8-10 ml / kg, gemodez or neocompensan 15-20 ml / kg, mafusol 8-10 ml / kg, refortan or infucol 6-8 ml / kg kg, reopoliglyukin 15-20 ml/kg. The total amount of injected solutions should approximately exceed the daily requirement by 1,5 times. Author: Kolesnikova M.A.
▪ General foundations of pedagogy. Lecture notes
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ Creation of an artificial uterus ▪ Biometric scanners for smartphones ▪ Microsoft wrote 200 MB of data into DNA
▪ site section Welding equipment. Article selection ▪ article Psychological help after situations with high danger to life. Fundamentals of safe life ▪ article What is weevil? Detailed answer ▪ article Conducting public events. Standard instruction on labor protection
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

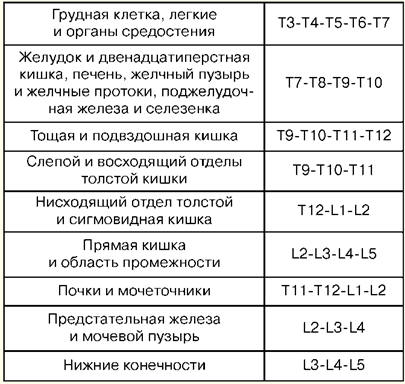






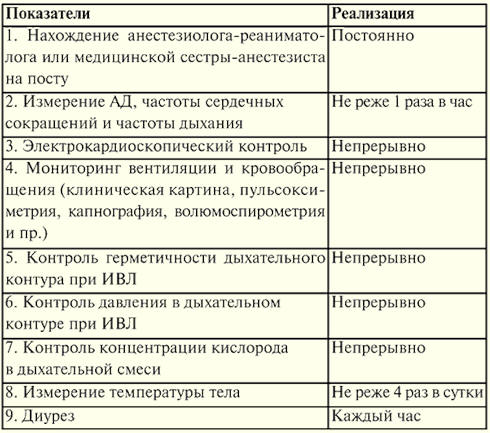




 See other articles Section
See other articles Section 