
|
|
ENCYCLOPEDIA OF RADIO ELECTRONICS AND ELECTRICAL ENGINEERING Sealed lead-acid batteries in amateur radio practice. Encyclopedia of radio electronics and electrical engineering
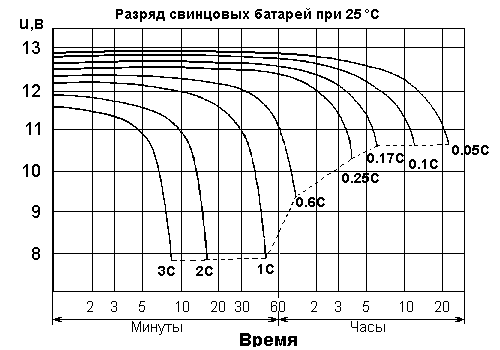
Encyclopedia of radio electronics and electrical engineering / Chargers, batteries, galvanic cells 1. First gingerbread, then whips ... Sealed lead-acid batteries (SLA) are the most affordable secondary (rechargeable) power sources. Affordable, in the current economy, means, firstly, the availability for sale of standard batteries with a voltage of 6V and 12V, with a capacity of one to a thousand Ah, and secondly, that for 1 evergreen cu. you can buy from 1.5 to 6 Wh of nominal capacity. The smaller number corresponds to small batteries, the larger number to large ones. What else is upside? Relatively slow self-discharge (no more than 5% of capacity per month at room temperature), relative durability subject to shallow discharge cycles. Lack of “memory” (typical of nickel-cadmium batteries). A constant “floating” recharge in standby mode is allowed (this is how car batteries work). Compared to lead-acid batteries with liquid electrolyte, sealed batteries naturally benefit in operational safety (no harmful fumes, operation in any position is acceptable). And also - a sealed battery is less critical to charging conditions, it is more difficult to kill it with an ill-fitting charge. The fact is that the gel electrolyte is selected in such a way that the battery is never fully charged (from a chemist’s point of view). Therefore, gas evolution does not occur during recharging, since there is simply no recharging. This does not mean that you can forget about controlling the charge mode. It is forbidden. More on this later. What's the downside? Firstly, low specific capacity - 25..35 Wh per kilogram of mass, or 60..100 Wh per liter of volume. Secondly, a significant reduction in battery life during deep discharge cycles, as well as during systematic discharge with high currents. Thirdly, there is a significant dependence of voltage and internal resistance on the cycle depth. 2. About premature old age Terminology: in practice it is customary to designate discharge intensity in the form of dimensionless "C units". 1C (one-tse) is numerically equal to the battery capacity when discharged with direct current for 20 hours. A full discharge is defined as a discharge of up to 1.8V per cell at room temperature (i.e. up to 5.4 and 10.8V for 6V and 12V batteries). The value of 1.8V was established empirically as the lower limit, when discharged below it with a current of 0.05C, irreversible premature aging of the battery begins. Thus, if it is determined experimentally for a battery that in order to discharge it from a fully charged state (20-2.1V per cell) to 2.3V per cell in 1.8 hours, a discharge current of 150 mA is required, then the nominal capacity of the battery is set to 3.0 A*h (=0.15A * 20h). Current intensity 1C for a given battery corresponds to a discharge current of 3A, 2C corresponds to a discharge current of 6A, etc. If you limit the discharge by reaching a given minimum voltage, the same 10.8V, it turns out that the real capacity at a current of 1C will be reduced by approximately half compared to the nominal one (see graph). But the threshold for irreversible aging at high discharge intensity (1C and above), on the contrary, is significantly reduced - to 8V.
Repeated discharge of the battery to voltages below the dashed line leads to battery failure In practice, SLAs operate in two modes - buffer and cyclic. In buffer mode, the battery is constantly connected to the charger. If there is voltage in the electrical network, then after charging the battery is exposed to the final charge voltage for a long time. The low current flowing through the batteries compensates for the battery's self-discharge and keeps the battery in a fully charged state at all times. In the event of a power outage, the battery is discharged to the load connected to it. The buffer mode of operation is typical for uninterruptible power supply systems of direct and alternating current, which are widely used for computers, communications and continuous production. And also - car batteries during regular use of the car. In cyclic operation, the battery is charged and then disconnected from the charger. The battery is discharged as needed. The cyclic operating mode is used when operating various portable or transportable devices: electric lights, communications equipment, measuring instruments. Battery manufacturers usually indicate in the list of technical characteristics what operating mode a particular battery is intended for. Therefore, if you decide to power the filament batteries in a tube amplifier, then this is a cyclic mode (how nice it is to know that you have been speaking in prose all your life...). But does this mean that you can simply discharge the battery to the maximum permissible lamps 5.7 or 11.4V? In fact, even though this mode is obviously safer than discharging to “emergency” 5.4 or 10.8V, if the battery is chosen incorrectly, it will lead to fairly deep discharge cycles, and thereby shorten its service life. Cycle depth discharge is defined as the ratio of ampere-hours actually delivered to the load to the ampere-hours corresponding to the discharge to the threshold of irreversible aging. The ampere hours in the denominator will coincide with the rated capacity only for a discharge intensity of 0.05C. In practice, it is the nominal capacity that is used as the denominator (especially since the constant discharge current is nothing more than an ideal approximation).
The depth of the cycle (if it is repeated from cycle to cycle) determines the life of the batteries. At 100% cycle depth, the SLA service life will not exceed 200-300 cycles. For reference, car batteries with liquid electrolyte rarely withstand more than 20 deep cycles. At 30% cycle depth, their number triples. The famous Optima guarantees survival for 100 zero-cycle cycles (the author has had such a battery for four years, but there hasn’t been a single deep zero-cycle cycle...). 3. Real life example Now let's count. Each channel of the amplifier contains a pair of 6C4C lamps (6V, 2A). A minimum operating time between charges of 8 hours must be ensured. In this case, the voltage should not fall below 5.7V (according to the lamp specifications), the cycle depth should not exceed 50%. From the last requirement it follows that the battery capacity is at least 32Ah per channel (= 2A * 8h / 50%). The discharge rate of such a battery is 0.06C (= 32A*h / 2). From the graph it follows that in 8 hours its voltage will drop to only 12.0-12.2V. There is stock! But only with a fresh battery. If you don’t forget to charge it on time, then after about 500 cycles (a year and a half of daily enjoyment), the voltage in 8 hours will drop to the same 5.7V, or worse... Be sure to set the automatic to turn off when there is insufficient voltage! By the way, 32A*h is suspiciously close to the capacity of a car battery (50-65 A*h). So for currents of 2A and higher, a maintenance-free car battery is a completely reasonable (in terms of price) alternative. They have problems with the environment and safety. On the other hand, if a large battery does not fit into the design, then you can completely safely parallelize several smaller batteries (preferably, but not necessarily, of the same series, same manufacturer, same “age” from the start of operation). Or maybe try the buffer (standby) mode to charge constantly, without any automation? Toggle switch up - the battery is discharging, the lamps are playing, toggle switch down - charging is in progress, the lamps... are disconnected from the batteries! Normal charging mode - charge with a constant voltage of 2.4-2.5V per jar, at the 6V battery terminals there will be up to 7.5V - the lamps will not last long (especially if the anode power is turned off). In buffer mode, battery life is highly dependent on temperature. The most favorable temperature for the battery is considered to be 15-20 degrees Celsius. An increase in temperature by 10 degrees reduces battery life by half. The figure shows a typical dependence of the service life on temperature for batteries with an estimated service life of 5 -7 years. Summary - do not put batteries in the same case with lamps, Pentiums, etc. hot objects. You may ask - what about under the hood in a car... well, firstly, a car battery is specially designed for a wide range of temperatures, and secondly, the heat capacity of the battery is so high that it is not easy to warm it up significantly, even under the hood.
In the above example, the service life of the incandescent battery at daily 50% cycles is one and a half years. Is it possible to do more? In real operating conditions of stationary batteries, it is necessary to take into account the reduction in battery life in the case of a large number of tested discharges. For 5-year batteries, the actual life will be no more than 3 years if the battery experiences an average of one 30% discharge per day or one full discharge per week. 4. Learn more about the charge The best battery charging mode for a small (not higher than 75%) discharge depth is constant charge voltage. Different manufacturers give slightly different values, but a generally accepted voltage is 2.4V per cell when cycling (14.4V for a 12V battery). In buffer mode, the voltage can be lower, 2.3V per cell. When charging a completely discharged battery, this mode leads to an initial current overload, so a combined current and voltage limiting mode is used. It is usually called IU charge mode. A discharged battery is first charged with a direct current, numerically (in amperes) not exceeding 0.1-0.3 of the nominal battery capacity (in ampere-hours). For example, for a battery with a capacity of 100 A*hour, the charge current should not exceed 10-30 amperes. As the battery charges, the voltage across the battery increases (at constant current). After the voltage on the battery reaches the final charging voltage, the charging current begins to decrease, keeping the voltage constant. The final charge voltage at a temperature of 20 degrees Celsius is 2.25-2.3 volts per battery cell. For a battery with a nominal voltage of 12 V (6 cells), the final charge voltage is 13.5-13.8 V. If the battery is operated at other temperatures, then to increase the battery life it is recommended to reduce the final charge voltage to 2.2-2.25 V/cell at a temperature of 40 degrees and increase voltage up to 2.35-2.4 V at a temperature of 0 degrees. The use of such temperature compensation of the charging voltage allows you to increase the battery life at 40 degrees Celsius by 15%. To fully charge a depleted battery, it is recommended to charge it for 24 hours. If a faster (within 8-10 hours) battery charge is required in the case of cyclic operation, the final charge voltage is increased to 2.4-2.48 V/el (at 20 degrees Celsius) and the charging time must be limited in accordance with the remaining charge of the battery before charging . Here is an example of similar instructions for a Fiamm GS battery (source - slt.ru): Constant voltage charger A relatively large current is applied during the initial charging phase of the battery. When the battery voltage reaches the set level, the charger switches from constant current mode to constant voltage mode. During this phase, the charging current begins to decrease to a minimum charging current level, known as the float current. The values given in the table are taken as standard. Standard values of electrical quantities for a charger with constant charging voltage
Notes: For batteries used in cyclic mode, it is recommended to use a sensor that allows the charging process to be interrupted when a preset voltage value is reached, or a timer. The temperature coefficient must be taken into account if the battery is charged at temperatures below +100From or above +300С Rapid charge system (only for batteries operating in cyclic mode)When accelerating battery charging, it is necessary to use devices equipped with a temperature compensation unit and a thermal fuse to prevent the battery from being undercharged at low temperatures or overheating at high ambient temperatures. The standard values of electrical quantities for the accelerated battery charging mode are given in the table:
Notes: The battery must have a thermostat or thermal fuse installed, or a timer must be used to stop the charging process in time. The maximum initial charging current for batteries with a capacity greater than 10 Ah must correspond to the following ratio: I = C maximum Pay attention to the last paragraph. He's worth it. Especially if many batteries are walled up in a poorly ventilated box, overheating is possible even with a normal (not accelerated) charge, although not catastrophic, but still shortening the life of the batteries. 5. Simple charger (slow charge IU) For charging small batteries, the most convenient standard circuit is based on the IC family LM117, LM 196, LM317 (142EN12, 1151EN1, 1157EN1). Source - "Microcircuits for linear power supplies", M, Dodeka, 1998, pp. 97, 122, etc.).
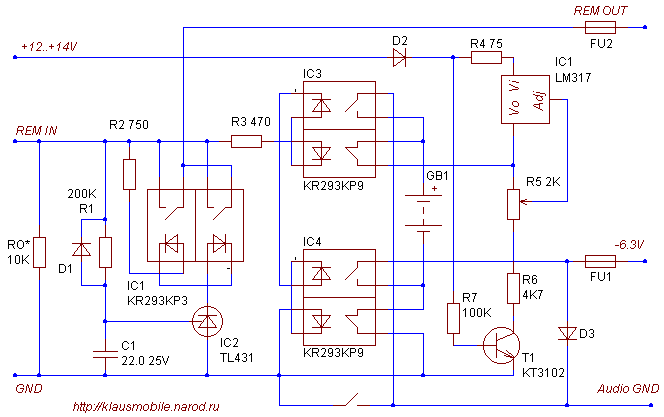
The current limiting threshold is set by R4 (taking into account the permissible current and power dissipation of the microcircuit). In practice, when the power supply for a specific type of battery is built directly into the equipment - no current limit adjustment is needed, you can completely eliminate the current limiting circuit (T2), transferring this function to the output resistance of the power supply filter. At high currents, it is more convenient to use discrete stabilizers with pass-through N-MDS or composite npn transistors controlled by an integrated stabilizer. The inconvenience of MIS - a relatively high threshold voltage - in low-power chargers is solved by increasing the voltage of the main (single) power source, in powerful ones (see figure) - by doubling the voltage. The ratings of the voltage stabilizer dividers (IC1) are indicated for 6V batteries, the ratings of the filter capacitances and current stabilizer resistors (T2) are for charging currents of no more than 2.5A, which is sufficient for batteries with a capacity of up to 10-15 A*h. Transformer for output voltage 9V xx, current 5A. Switchable shunts in the T2 base-emitter circuit set the maximum charge current. Diode D11 - a Schottky diode with a current of at least 10A - protects against battery reverse polarity. The setup comes down to setting the stabilization voltage at a load equivalent of 10 Ohms (R6) and selecting shunts R5. 6. Negative voltage source in the car For powering crossovers, etc. devices on an op-amp with direct coupling, you can supply a simple pulsed negative voltage source. Or better yet, a battery. Much better! But this battery should not be 12, but 6 Volt. Let me explain. Most likely, this battery will supply current almost always when the engine is running. And it can only charge while parked. But it is impossible to charge a 12V lead battery from another 12V battery. This is not even a buffer regime, but a hunger strike. You need a generator that produces 14V, but where can you get one, in the parking lot... To power a crossover with a current consumption of 20mA, a 6V, 1.2Ah battery (the size of a little more than a pack of cigarettes) is sufficient. Charge mode IU (200mA, 7.2 V). When the REMOTE signal is turned off, the battery is constantly charged from the on-board network (minus to ground, plus to the stabilizer output - the state of the optocouplers is as shown in the diagram). When the REMOTE signal is turned on, the battery is switched positive to ground and negative to the load (op-amp power bus). The charge current is limited by resistor R3 at 75 mA. A fully charged Fiamm 10121 battery in this mode takes approximately 15mA from the on-board network at room temperature. The R7-T1 chain blocks the discharge of the battery to the R5-R6 divider when disconnected from the on-board network (assuming, of course, that REM IN is removed and the battery load is disconnected). Current consumption via REMOTE bus 20mA. Timer D1-C1-R1-IC1-IC2-FU1 delays the transmission of the REM IN signal to the output by 2 seconds. Resistor R0 is needed only to discharge the timer capacitance; in practical circuits it can be eliminated or replaced with an indicator circuit with an LED. Diodes D1-3 - any for direct current 1A. Optocouplers KR293KP9A, KR293KP3A can be replaced with any MIS optocouplers with a current of at least 200mA (293KP with the letter A). When switching the battery with a KR293KP9A optocoupler with “anti-phase” switches in one case, I did not observe any through current during switching; when replacing it with other optocouplers, you should make sure that there is none. Fuses FU1, FU2 are self-restoring fuses with an operating current of 200 mA. In the power filter at the output of the -6V source, you should limit yourself to a minimum capacitance so as not to overload the optocouplers during switching; by the way, they add 10 Ohms to the output resistance of the battery). Series 293 is not for ampere currents! This is for "adult" relays. This is the topic of the next project - a fully battery powered DAC... but it's too early for that... Publication: klausmobile.narod.ru
Machine for thinning flowers in gardens
02.05.2024 Advanced Infrared Microscope
02.05.2024 Air trap for insects
01.05.2024
▪ Prototype anode for sodium-ion batteries ▪ New ICs for power factor correction in rectifiers ▪ Nanomechanical microchip sensor with ceramic coating ▪ Graphene based infinite energy generator ▪ SpaceX Crew Dragon spacecraft successfully returned from the ISS
▪ site section Parameters, analogues, marking of radio components. Article selection ▪ article Photo and video shooting on a hike. video art ▪ article Who made the first sails? Detailed answer ▪ article Make a hygrometer. Children's Science Lab ▪ Clay's article. Simple recipes and tips ▪ article Two bouncing rubber bands. Focus secret
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua | |||||||||||||||||||||||||||||






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese




 Leave your comment on this article:
Leave your comment on this article: