
|
|
ENCYCLOPEDIA OF RADIO ELECTRONICS AND ELECTRICAL ENGINEERING Comparative characteristics of galvanic elements of size AA. Encyclopedia of radio electronics and electrical engineering
Encyclopedia of radio electronics and electrical engineering / Chargers, batteries, galvanic cells Today in stores and markets you can find many different galvanic cells. Which ones to choose? This article will help you make the right decision. Galvanic cells and batteries are widely used to power various electronic equipment. AA size elements are the most widely used. On the shelves you can find similar products from different companies, mainly two electrochemical systems: saline and alkaline. More recently, Energizer has launched 1,5 V AA lithium batteries. The most important characteristic of a galvanic cell - capacity (the amount of electricity that it is able to give to the load) - is almost never indicated on the label. The buyer is left to focus on television commercials about cells that “work up to ten times longer than conventional salt”, or take the word of Energizer, which claims that its new AA lithium e2 cells last five times longer than conventional alkaline ones [1]. Moreover, it remains not entirely clear which elements are called "ordinary". In order to quantitatively compare the parameters of elements of different electrochemical systems, it is necessary to test them under the same conditions. Such tests were carried out with three types of cells: salt Philips Long Life (emf of a "fresh" cell - 1,65 V), alkaline Duracell Ultra M1,62 (2 V) and lithium Energizer e1,8 (15 V). Each of them was loaded with a 100 ohm resistor, which corresponds to an initial discharge current of approximately 1 mA. For elements of size AA, such a load current is typical. The discharge was carried out in cycles of several hours a day, which corresponds to real operating conditions. This explains the voltage spikes in the discharge curves shown in Figs. XNUMX. The blue curve corresponds to the salt element, red - alkaline and green - lithium. During the "rest" the voltage on the element of any type increased slightly, but after the load was connected, it quickly decreased to the minimum in the previous cycle. The dots mark the values of the EMF of the elements - the voltages on them without load.
If we take as a criterion for the complete discharge of the cell a decrease in the voltage at its load to 0,9 V, the experimentally determined capacity of the salt cell was 1 Ah, the alkaline cell - 2,9 Ah, and the lithium cell - 3,5 Ah. Consequently, there is no need to talk about any five- or ten-fold differences in the capacitance of the elements of different electrochemical systems. On fig. 2 shows another series of curves.
They show how the internal resistance of the elements changed during the discharge process. The correspondence between element type and curve color is the same as in Fig. 1. The values of the internal resistance R, were calculated by the formula
where E is the EMF of the element; U - voltage under load; RH is the load resistance. The internal resistance of the salt and alkaline elements increases monotonically as they are discharged. And the resistance of lithium, having sharply decreased at the beginning of the discharge, remains practically unchanged until its very end, and then increases just as sharply. Of course, the experiments carried out cannot be called exhaustive. The capacity of an element is not a strictly fixed value, it depends on many external factors. For different elements, its maximum can be achieved under significantly different discharge conditions. To take all this into account, a very large series of experiments, unrealistic in amateur conditions, would have to be carried out. However, let's try to check the obtained results by calculation. In order to theoretically estimate the maximum possible capacity of elements of various electrochemical systems, it is necessary to know the chemical composition of their electrodes, electrolyte, and the chemical reaction taking place in the element. In saline and alkaline cells, the cathode is zinc, and the anode is manganese dioxide. It is for this reason that such elements are collectively called manganese-zinc. But the electrolyte in them is different: salt (usually ammonium chloride) or alkali (potassium hydroxide). According to [2], the reaction occurs in a salt manganese-zinc element
and in alkaline
There is no reliable information about the material of the electrodes and the chemical reaction in the lithium cell. We can only assume that the electrodes are lithium and manganese dioxide, and the electrolyte is a solution of lithium perchlorate in propylene carbonate. If this conjecture is correct, according to [2], the lithium cell undergoes the reaction
Using Faraday's law, we obtain an expression for determining the capacitance of a galvanic cell C, Ah:
where m is the mass of the reacting substances F = 96,5-103 C/g-eq is the Faraday number; n - valency (for salt and alkaline galvanic cells - 2, for lithium - 1); M is the total molecular weight of the reactants. We weigh galvanic cells of size AA: salt - 17 g, alkaline - 24 g, lithium - 15 g. ) is negligible and can be neglected. We calculate the total molecular weight of the reacting substances from the above equations of chemical reactions: for salt - 346 g, for alkaline - 257 g, for lithium - 94 g. Substituting numerical values into the formula, we obtain the maximum possible capacity of the salt element - 2,6 Ah , alkaline - 5 Ah, lithium - 4,3 Ah. The differences between the calculated capacitance values and the measured ones can be explained by rather rough assumptions made in the calculation. So five- and ten-fold differences were not found. The theoretical capacity of an alkaline cell is about twice that of a salt cell, and lithium has no advantage over alkaline in this respect. This is consistent with the experimental results. Based on the results of all the work done, we can conclude the following: 1. Lithium galvanic cells have the most stable voltage, the lowest internal resistance, which practically does not depend on the degree of discharge, and the largest, although not much, capacity. It is preferable to use them to power equipment with a large current consumption, as well as in devices that automatically turn off when the power supply voltage drops (for example, digital cameras). 2. Alkaline cells have a capacity comparable to that of lithium, and are also capable of delivering high current to the load, but at a lower voltage. They are best used in devices with an average current consumption without automatic voltage control. In many cases, alkaline cells are preferred over lithium because they are three to four times cheaper. 3. Salt cells have the smallest capacitance and the highest internal resistance. It is advisable to use them in equipment with low current consumption. Literature
Author: I.Podushkin, Moscow
Machine for thinning flowers in gardens
02.05.2024 Advanced Infrared Microscope
02.05.2024 Air trap for insects
01.05.2024
▪ NASA tested a space nuclear reactor ▪ 3-atom thick LED for ultra-thin flexible screens
▪ section of the site Medicine. Selection of articles ▪ Bluebeard article. Popular expression ▪ article When did dentistry appear? Detailed answer ▪ article The functional composition of Onwa TVs. Directory ▪ article SE on lamps 6N30P and 6E5P. Encyclopedia of radio electronics and electrical engineering
Comments on the article: a guest Explanatory article, thank you.
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese






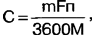
 Leave your comment on this article:
Leave your comment on this article: