Using an optocoupler in the feedback circuit of a voltage stabilizer or charger. Encyclopedia of radio electronics and electrical engineering

Encyclopedia of radio electronics and electrical engineering / Chargers, batteries, galvanic cells
 Comments on the article
Comments on the article
A simple, low-cost circuit that simultaneously functions as a stabilizer and charger for low-capacity batteries can be assembled without the use of complex voltage sensors. In this circuit, the diode (emitter) of the optocoupler, included in a simple feedback circuit, perceives changes in the output voltage. The circuit generates a stabilized output voltage of 12,7 V at a current of 50 mA and can be used to charge batteries while maintaining the current and voltage limits, which are quite easy to change.
The optocoupler is the optimal device in terms of its application as a voltage sensor. The diode perceives the output voltage without loading the circuit and without violating the normal operating mode, and the voltage across it does not change and has a relatively small value for any changes in the charging or load currents.
As shown in the diagram, the diode bridge and capacitor C1 rectify and filter the AC input voltage. Let's assume that the circuit works as a charger.
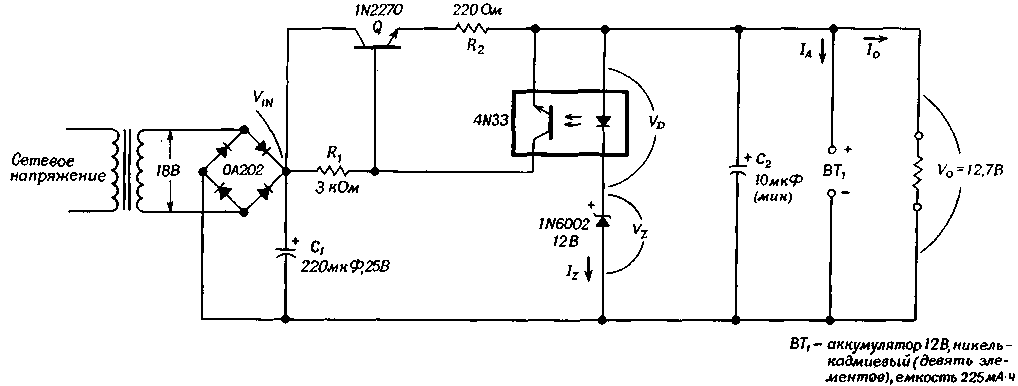
(click to enlarge)
When the battery is not fully charged, the voltage on it is below 12,7 V (Vz + Vd). This voltage is set by selecting an appropriate silicon zener diode, which is in series with the optocoupler diode. In this case, the 1N2270 series transistor turns on and passes current to the battery. The 1A current is limited mainly by a 220 ohm resistor.
When the battery voltage exceeds (Vz+Vd), the zener diode turns on and current Iz flows through the optocoupler diode, turning on the phototransistor and turning off the series transistor Q. In the absence of a battery, when the circuit is in regulator mode, the current enters the load at a voltage of 12,7 B. In this case, of course, the output current depends mainly on the load resistance.
The ripple voltage is 25 mV in stabilization mode and 1 mV in charging mode. The circuit provides stabilization of 30 mV / V with voltage changes and 8 mV / mA with load changes in the range from 5 to 30 mA. Both parameters can be improved by replacing transistor Q with a compound transistor.
The output voltage and current can be set by appropriate selection of resistors R1 and R2 and a zener diode. In addition, the resistances of the resistors can be determined if the battery capacity (C) in milliamp-hours and the input voltage (on capacitor C1) are given.
It was experimentally found that for the best operation of the circuit, the current Ia should be equal to 0,25 C. This ratio is typical for batteries that consume a relatively large charging current. Assuming the input voltage Vin much greater than the base-emitter voltage drop of transistor Q, we get
R2>Vin/0,25C-R1/hfe,
where hfe is the DC gain of transistor Q. To find the minimum value of R2, assume that Io is known, therefore,
R2<(Vin-Vo/Io.
This equation is valid provided that Io>Iz.
The value of R1 depends on the minimum value of Io corresponding to the off state of the transistor Q. It can be shown that
R1>(Vin-Vo)/0,02C.
This equation assumes that the minimum value of Io is about 0,02C - a ratio determined experimentally and not theoretically.
Author: LA Cherkason; Publication: N. Bolshakov, rf.atnn.ru
 See other articles Section Chargers, batteries, galvanic cells.
See other articles Section Chargers, batteries, galvanic cells.
 Read and write useful comments on this article.
Read and write useful comments on this article.
<< Back
 Latest news of science and technology, new electronics:
Latest news of science and technology, new electronics:
Air trap for insects
01.05.2024
Agriculture is one of the key sectors of the economy, and pest control is an integral part of this process. A team of scientists from the Indian Council of Agricultural Research-Central Potato Research Institute (ICAR-CPRI), Shimla, has come up with an innovative solution to this problem - a wind-powered insect air trap. This device addresses the shortcomings of traditional pest control methods by providing real-time insect population data. The trap is powered entirely by wind energy, making it an environmentally friendly solution that requires no power. Its unique design allows monitoring of both harmful and beneficial insects, providing a complete overview of the population in any agricultural area. “By assessing target pests at the right time, we can take necessary measures to control both pests and diseases,” says Kapil ... >>
The threat of space debris to the Earth's magnetic field
01.05.2024
More and more often we hear about an increase in the amount of space debris surrounding our planet. However, it is not only active satellites and spacecraft that contribute to this problem, but also debris from old missions. The growing number of satellites launched by companies like SpaceX creates not only opportunities for the development of the Internet, but also serious threats to space security. Experts are now turning their attention to the potential implications for the Earth's magnetic field. Dr. Jonathan McDowell of the Harvard-Smithsonian Center for Astrophysics emphasizes that companies are rapidly deploying satellite constellations, and the number of satellites could grow to 100 in the next decade. The rapid development of these cosmic armadas of satellites can lead to contamination of the Earth's plasma environment with dangerous debris and a threat to the stability of the magnetosphere. Metal debris from used rockets can disrupt the ionosphere and magnetosphere. Both of these systems play a key role in protecting the atmosphere and maintaining ... >>
Solidification of bulk substances
30.04.2024
There are quite a few mysteries in the world of science, and one of them is the strange behavior of bulk materials. They may behave like a solid but suddenly turn into a flowing liquid. This phenomenon has attracted the attention of many researchers, and we may finally be getting closer to solving this mystery. Imagine sand in an hourglass. It usually flows freely, but in some cases its particles begin to get stuck, turning from a liquid to a solid. This transition has important implications for many areas, from drug production to construction. Researchers from the USA have attempted to describe this phenomenon and come closer to understanding it. In the study, the scientists conducted simulations in the laboratory using data from bags of polystyrene beads. They found that the vibrations within these sets had specific frequencies, meaning that only certain types of vibrations could travel through the material. Received ... >>
 Random news from the Archive Random news from the Archive Sodium-ion battery
12.03.2015
Chemists have made a sodium-ion battery that works just as well as the lithium-ion battery we are used to.
A few years ago, it was suggested that it is time for humanity to think about an imminent shortage, but not about the oil and gas one, which we are usually afraid of, but about the shortage of an alkali metal - lithium. In our life there are more and more electronic devices and all kinds of gadgets. And all of them, from a mobile phone to an electric car, use electrical energy stored in batteries. Most of these are lithium-ion batteries. Today it is the most common type of rechargeable batteries. And although we are unlikely to see wars over lithium deposits in the near future, its cost may increase. And this means that it is time to think about cheaper batteries that would use other cells. Developers are betting on the closest relative of lithium in the periodic system - sodium, as a much more common and inexpensive metal.
Why can't you just take and replace lithium in a battery with sodium? It's all about atomic size. Although lithium and sodium are very similar in their chemical properties, the sodium atom is significantly larger than the lithium atom. And it turns out to be critical for the operation of the battery. A lithium battery has two electrodes, one made of carbon or graphite and the other made of a metal oxide such as cobalt. Lithium ions serve as a charge carrier between the electrodes, which is why, in fact, they are called lithium-ion batteries. During recharging, lithium ions are released from the metal oxide electrode and move to the second electrode, which is made of carbon.
The size of lithium atoms is such that they can easily be integrated into the structure of the electrode. This process is called intercalation, during which metal ions "squeeze" between the atomic layers of graphite. During discharge, the reverse process occurs - lithium ions leave the graphite electrode and return to the second electrode.
The key point of this electrochemical process is just the incorporation of ions into the electrode. The faster and easier it passes, the greater the instantaneous power can be. If the process is slow, the battery will not be able to provide the current needed to operate the device. This is precisely the difficulty in developing a sodium-ion battery. A carbon electrode is not suitable because sodium ions, due to their size, are extremely reluctant to integrate into the graphite structure.
That's why electrochemists are looking for electrode materials that are suitable for conventional electronics. After all, it is possible to make a battery on sodium ions, and it will work, the whole point is that it will not be as small, capacious and powerful as lithium. But it is power and size that are the most important parameters for mobile devices.
A team of researchers led by Professor Yong Lei from the Technical University of Ilmenau in Germany came up with a material that can be used to make an electrode in a sodium-ion battery, so that it will not be inferior to lithium in terms of power and capacity.
First, the chemists analyzed what properties the electrode material should have in order to ensure the effective introduction of sodium ions. The choice fell on conjugated aromatic compounds of the trans-stilbene class. They have the ability to transfer charge, are stable when charging and discharging the battery, and form intermolecular layers between which sodium can easily be introduced.
Chemists tested how well an electrode made of such a material would work and it turned out that at an average current density of 1 A / g, the capacity would be 160 mAh / g, which is in no way inferior to lithium-ion batteries. The battery also performed well in the endurance test, retaining 70% capacity after 400 charge-discharge cycles. And although the commercial implementation of the project is still far away, the results achieved indicate that sodium-ion batteries have the right to life and can, in principle, replace the already familiar Li-ion batteries.
|
 Other interesting news:
Other interesting news:
▪ Baseus portable battery 180 mA
▪ The coldest cubic meter in the universe
▪ Processing moon dust into oxygen
▪ Children's smart watch Garmin Bounce
▪ Makeup and pop culture made people afraid of clowns
 News feed of science and technology, new electronics
News feed of science and technology, new electronics
 Interesting materials of the Free Technical Library:
Interesting materials of the Free Technical Library:
▪ section of the site for the radio amateur-designer. Article selection
▪ article And everything that he saw before him, he despised or hated. Popular expression
▪ article What does historical science study? Detailed answer
▪ article Working on a sewing machine. Standard instruction on labor protection
▪ article Alternative energy from waste. Encyclopedia of radio electronics and electrical engineering
▪ article Pinout of common bipolar and field-effect transistors. Encyclopedia of radio electronics and electrical engineering
 Leave your comment on this article:
Leave your comment on this article:
 All languages of this page
All languages of this page
Home page | Library | Articles | Website map | Site Reviews

www.diagram.com.ua
2000-2024







 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

 Leave your comment on this article:
Leave your comment on this article: