
|
|
ENCYCLOPEDIA OF RADIO ELECTRONICS AND ELECTRICAL ENGINEERING Cleaning up finds. Encyclopedia of radio electronics and electrical engineering
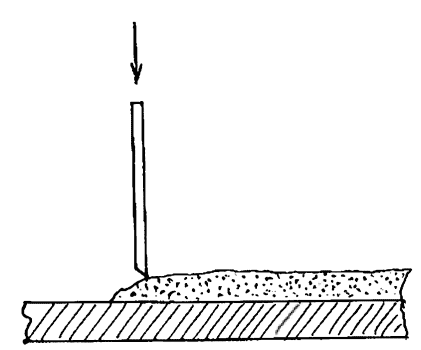
Encyclopedia of radio electronics and electrical engineering / metal detectors Objects found in the ground are usually covered with a crust of oxides. If you doubt whether you can remove them, then it is better not to do this. In some cases, cleaning is a problem that should be given the most serious attention, since a valuable find is very easy to spoil or greatly depreciate in the process of unskilled cleaning, when accelerated methods of removing oxides are used. It is better to contact specialists who, using complex chemicals and even a scalpel, will remove the slightest traces of corrosion, as a result of which the product looks like new, allowing you to see the smallest details of the image, for example, on a Roman coin. However, the antiques market and collectors do not welcome this type of cleaning, preferring to preserve the natural patina on the products. For those who still want to try to clean the found products themselves, below are some of the methods and chemicals used to clean various metals and alloys. Mechanical cleaning This method involves the removal of surface deposits manually. Loose corrosion products can be removed with brushes. For this, toothbrushes of various hardness are used. Typewriter type brushes have worked well, but these are now unfortunately hard to find. For large iron products, wire brushes can be used. Solid oxide layers can be chipped off with a sharp tool made from ordinary sewing needles, the end of which is sharpened like a chisel. The corrosion layer is chipped piece by piece. In this case, the tool should be held vertically and then ensure that it does not touch the base metal. The process is slow, but the results can be very good. It is very useful to use a table magnifier during such cleaning.
Rice. 60. Mechanical cleaning - chipping off oxides with a sharpened needle There are electric tools that speed up the cleaning process. These are small-sized drills, where various brushes are installed instead of a drill, as well as vibrating engraving machines, which make it easy to chip off solid oxides from the product, again using sewing needles or special nozzles as tools.
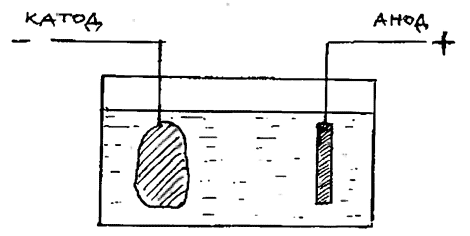
For cleaning large batches of homogeneous products (for example) modern coins, tumbling drums can be used, in which the objects to be cleaned rotate together with a filler of wooden, plastic or metal elements in soapy water for several hours. Electrolytic cleaning Equipment for electrolytic cleaning consists of a transformer, a rectifier and two electrodes - a cathode (-) and an anode (+). A stainless steel plate is connected to the anode, and a clamp or wire frame is connected to the cathode, holding the object that must be cleaned of corrosion. Both electrodes are lowered into a glass or plastic container into which the electrolyte is poured. This is usually water with a small amount of table salt and a suitable cleaning agent such as caustic soda or citric acid. When the current is turned on, the gradual removal of corrosion products begins. From time to time, the current is turned off and the surface of the product is inspected. When the oxides lose their hardness, the process can be stopped and further cleaning can be carried out mechanically. Electrochemical cleaning This is another kind of electrolytic cleaning, in which chemical reactions occur without the use of an external power source. The cleaned item is placed in a container with the solution and a suitable metal powder is added, usually zinc to the caustic soda solution or aluminum to the sodium carbonate solution.
As a result of a chemical reaction, hydrogen is released, which reduces oxides on the product. Chemical cleaning The product is immersed in a solution of suitable chemicals, which can be either acids or alkalis. They dissolve corrosion products on the product. The more concentrated the solutions, the faster the reactions go and, accordingly, the cleaning process is more difficult to control. Iron and its alloys Iron was used for the manufacture of products exclusively by forging. Casting was not used due to the high fragility of the cast products. Weapons and tools, in particular axes and knives, were made by forging a floor of variously processed metal. At the same time, a hard steel cutting core was surrounded by softer iron, due to which the cutting edge self-sharpened in the process, like beaver teeth, and the tool was always sharp. Unfortunately, iron and steel corrode easily and are the most difficult to clean and preserve. Corrosion of iron is a complex process in which it is covered with a thick layer of iron oxides or rust. Sometimes this process ends with the complete replacement of the metal with oxides, although outwardly the product retains its original shape. This can be checked with a magnet (oxides are not attracted by a magnet). If you decide to clean an iron object, the method will depend on the state of the oxides. Small items such as arrowheads may have very little of the original iron left. Therefore, they can be improved by removing the bumpy rust formations with a file, thus preserving the shape of the tip. Larger objects (horseshoes, cannonballs) can be subjected to more severe cleaning, such as removing rust by chipping it off with a hammer or chisel. Scale from such objects is easily removed by heating the product to red heat and then lowering it into water or oil. Mechanical rust removal methods are preferred when cleaning iron objects. Small rust is easily removed with a steel brush. Thicker layers of oxides can be peeled off with a file or an abrasive wheel, but without forgetting the original shape of the object. During chemical treatment, oxides can be removed by immersing the product in solutions of mineral and organic acids with the addition of 1-2% acid corrosion inhibitor - urotropine, tannin, pyrocatechol, hydroquinone, metol. The most active is a solution containing 35% orthophosphoric and 5-10% hydrochloric acid. After acid cleaning, it is necessary to thoroughly rinse the surface of the cleaned metal and subject it to preservation using a corrosion inhibitor, preferably benzotriazole. In some cases, the electrolytic method for removing oxides is preferable. To do this, use a 12V power supply (battery charger). The positive wire is connected to a stainless steel plate (spoon, for example), the negative wire is connected to the item to be cleaned. As an electrolyte, a solution of table salt is used (3 tablespoons per 1 liter of water). The distance between the product and the stainless steel plate is 3 cm. As the electrolysis proceeds, the water turns brown and is changed every 2-3 hours. After cleaning, rinse the product well, dry and coat with wax, varnish or petroleum jelly to prevent further oxidation. To clean the surface of iron from corrosion products, there are more complex methods that only enthusiasts can perform at home. Such methods include, for example, reduction in a low-temperature gas plasma. This method can be used to restore items made of completely or almost completely corroded iron, as well as items inlaid with gold or silver. In addition, thermal reduction of oxidized iron using carbon monoxide or hydrogen is used. In any case, an iron object cleaned of corrosion should be treated with some preservative material, otherwise it will begin to oxidize again. The first signs of such oxidation are the so-called "sweating", when small drops of rusty water appear on the surface of the product, after a while cracks form, the metal begins to delaminate and fall off. As a rule, this process is due to the presence of chlorides remaining in the corrosion products. To neutralize chlorides, the product is boiled in a 5% solution of caustic soda dissolved in distilled water, changing the solution several times. After that, the product is boiled in clean distilled water and dried for several days in an oven. Finally, the product is immersed in acetone, which helps to remove residual chlorides, and after final drying, immersed in molten wax for 1 hour. Excess wax is removed with a cloth and a hair dryer used to dry the hair. Finally, the product is covered with some kind of transparent varnish. Phosphating is widely used in restoration practice for ferrous metals as one of the reliable ways to protect the metal surface. Depending on the composition of the solution, the color of the phosphate layer can vary from colorless to black. Phosphating can be used to preserve iron objects with significant layers of corrosion products. Crystalline or amorphous layers of phosphates form on the surface, protecting the metal from further corrosion. Copper and its alloys Copper was used either in pure form or in the form of alloys. An alloy of copper with tin is bronze, an alloy with zinc is brass. Lead and other metals were also introduced into the alloys, giving the alloy other properties and appearance. Coins and products made of copper alloys after a long stay in the ground, as a rule, are oxidized. Copper oxides are mainly composed of copper carbonate and have a greenish color. However, the corrosion products can be more complex and contain many other elements that give the oxides a different look and can cause them to become unstable. As a rule, ancient coins and relics with a smooth green dense patina are much more highly valued by collectors than those that have been cleaned to a shiny metal or even more so polished. This means that cleaning in most cases of such objects is not required, except when they are covered with a thick, loose crust of oxides that hides details. In this case, it is best to use mechanical cleaning. Removal of corrosion products by chemical or electrochemical means may remove the patina or cause it to become unstable, leading to subsequent corrosion. Loose traces of corrosion are removed using a dental instrument used in dental treatment, including a drill. It should be borne in mind that products made of copper alloys were often coated with gold or silver, which must be taken into account when cleaning them mechanically. Separate hard areas of corrosion usually soften when the product is kept in olive oil for 3-5 days. Iron corrosion spots on copper alloys can be dissolved by topically applying a solution of disodium salt of ethylenediaminetetraacetic acid (Trilon-B). In some cases, when a thin layer of oxides covers products coated with gold or silver, it is easy to damage this coating during mechanical cleaning. In this case, it is recommended to use a solution of sodium hexametaphosphate. This chemical slowly dissolves the copper oxide layer. In the process of processing, the product should be removed from the solution from time to time and washed in water with a soft brush, observing the progress of the process. This chemical is especially useful when a layer of oxides has formed over the patina. The concentration of the solution should be 5-10%, a slight heating will speed up the reaction. Prolonged immersion in this solution can lead to the complete removal of oxides to pure metal. This solution allows you to remove lime deposits, adhering sand and clay. They soften and are relatively easy to clean with a stiff brush. Processing is significantly accelerated when using a hot 20% solution of sodium hexametaphosphate (40-50 ° C). The surface of copper and copper alloys is well cleaned with 5-10% solutions of citric and acetic acids, but after treatment in these solutions, the products must be thoroughly washed. One of the ways to clean products made of copper and copper alloys is to boil them in sunflower or other oil.
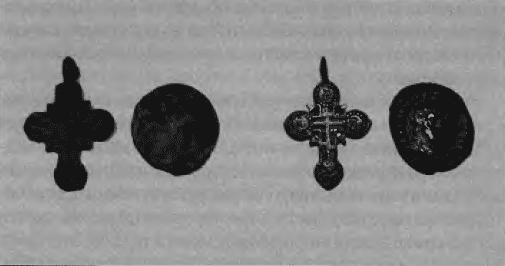
Rice. 63. Cleansing results At the same time, oxides soften and are easily removed with a brush, however, the products themselves often become black, which is not always desirable. To clean bronze with gilding, neutral and alkaline solutions of Rochelle salt are used. Rochelle salt does not react with copper oxides, but removes only salts and their hydrates. In some cases, electrolytic and electrochemical methods can be used to clean products. Electrolytic cleaning is carried out in a 5% citric acid solution at room temperature and a current density of 3-5 A/dm2. A negative wire is connected to the product, a stainless steel plate is used as the anode. From time to time, the product is removed and washed, observing how the cleaning is going. Perhaps, when some of the oxides are removed, further cleaning should be carried out mechanically. Electrochemical cleaning consists in applying a paste of powdered zinc, aluminum or magnesium to the product in a 10-15% caustic soda solution. The hydrogen released during the reaction contributes to the reduction of copper salts and oxides to metal. Bent objects made of copper and its alloys can be straightened by heating them in the flame of a gas burner. However, you should not overheat too much, otherwise the product may undergo significant oxidation and simply burn out. The appearance of some products can be improved by patina. The patina may be loose or coarse. If the color of the patina is too light or uneven, a colored wax treatment is useful. You can use multi-colored shoe polish (green, brown or black). Ordinary beeswax is also used as protective varnishes. If you had to clean the product to a clean shiny metal, then to improve the appearance, you can restore the patina by immersing the product in appropriate solutions. Many products made of copper alloys remain stable after being removed from the ground and do not require special treatment for their preservation. Waxes or protective varnishes can be used for additional protection and to enhance the appearance. Sometimes individual small areas of powdery green oxides are visible on the products, which come to the surface from the depth of the product. These oxides are due to the presence of chlorides contained in the corrosion products, which can be activated if the object's environment changes. This can also happen when the object has been chemically or electrochemically treated. Having arisen, such corrosion can progress and, if not paid attention to, can lead to the complete destruction of the product. The only way to deal with this disease is to completely remove the affected areas. This can be achieved by completely removing oxides from the product down to bare metal, or by removing oxides from the affected areas only with a dental instrument. If any traces of such oxides remain, the process will proceed further. To preserve the product after removal of copper oxides, it is recommended to treat it in benzotriazole. Before this, the product should be degreased in alcohol or acetone, after which it should be kept in a solution of caustic soda for a long time. After that, the product is dried and immersed for several days in a 5% alcohol solution of benzotriazole. After that, it is thoroughly dried and any sediment that appears on it is removed with a brush. Then several layers of protective varnish are applied to the product. Silver and its alloys Silver, as a rule, is alloyed with other metals, most often with copper. Often, silver items are coated with gold, and silver itself is used to cover items made of bronze, copper and other alloys. Sometimes silverware is decorated with silver niello applied to an engraved design. The main product of silver corrosion is silver sulfide, in the form of a thin black film, and copper carbonate, which forms a green crust. Less typical, but more difficult to clean, is silver chloride, which is a gray coating on metal. Copper carbonate is easily removed by immersion in a 5% solution of citric or sulfuric acid. When copper carbonate stains are observed on a piece, it is necessary to decide whether the piece is made of silver with a high copper content or is only covered with a layer of silver. In the latter case, when immersing the product in acid, you can ruin the item by dissolving the coating. Therefore, if coating is suspected, test a small area first with a drop of very dilute acid. Silver chloride is much more difficult to remove. It forms a strong film that grows inside the metal. Such a product can be cleaned by electrolysis using caustic soda as an electrolyte. After such treatment, it is necessary to boil the product in distilled water, changing the water several times, and then dry it at a temperature of 105°C. In some cases, the patina is formed by zinc chloride. If it has a beautiful shade, then it is even desirable, especially on coins. To remove the patina, you can use the following method. Wrap the product in aluminum foil, put in a glass jar. Add a little soda and pour hot water into the jar. After a while, when the bubbles stop, rinse the product in water. If required, repeat the process. If the product is not very deformed, it can be straightened without heating. However, some thin coins become very brittle over time and may break when bent. Therefore, with a strong deformation of the product should be annealed. Products made of high-grade silver do not require conservation. Alloys with a low silver content are better preserved when covered with a protective varnish. Gold and its alloys Gold was rarely used to make coins and items. Natural gold sometimes contains a high percentage of silver (up to 50%). This alloy is called electrum. For practical use, silver or copper is added to gold, sometimes both metals. As a result, alloys are obtained that are similar in appearance to pure gold, but significantly surpass it in hardness and wear resistance. Both pure gold and most of its alloys have high corrosion resistance and, being in the ground for centuries, do not change their appearance. Low-grade gold items and coins are often covered with patina, that is, they lose their luster and look dull and gray. So, some Taman gold coins can be mistaken for lead by an inexperienced eye. Items made of high-grade gold, extracted from the ground, as a rule, are as shiny as if they were buried yesterday. So the most you can do is wash them in warm soapy water with a soft toothbrush. Adhering lumps of earth are removed with a wooden toothpick. In general, ancient gold products have a matte, slightly orange color, which is lost by any attempt to rub the product with a piece of cloth or leather. Gold items are easily deformed. If they are not very badly bent, they can be corrected without heat. With significant deformation, the product should be heated in a flame to a dark red heat, and then lowered into water. The process should be repeated after each 20-30° bend. Unfortunately, this firing results in a loss of gold color, which can be restored in dilute sulfuric acid. However, the noble patina is much more difficult to restore. Gold does not require any special conservation. Tin, lead, and its alloys Tin and lead, along with copper, gold and silver, are metals that have been used by man since ancient times. Products from these metals are easy to manufacture by casting. However, they were rarely used in their pure form due to their low hardness. Much more often they were used in the form of alloys with other metals - bismuth, zinc, antimony, etc. Lead products (bullets, amulets, seals) are covered with a film of white oxide, which is stable under normal conditions. Therefore, it should not be removed. However, if the object has small details (printing) or a fine pattern, then the oxides can be removed in a 5% solution of Trilon-B. Oxides from products made of tin and its alloys with lead are removed with difficulty, accompanied by corrosion of the product. Therefore, it is often enough to simply wash the item in hot water and soap, and then wipe it with a flannel. Author: Bulgak L.V.
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ Logitech G604 Lightspeed Gaming Mouse ▪ Global satellite map of the rainforest ▪ Antidepressants for alcoholism ▪ Hydrogel for flexible electronics
▪ site section Electrician's tool. Article selection ▪ article Physiological foundations of labor. Fundamentals of safe life ▪ article Which birds helped the Japanese and Chinese in fishing? Detailed answer ▪ article junior consultant. Job description ▪ article Audio Frequency Generator. Encyclopedia of radio electronics and electrical engineering ▪ article The easiest phone. physical experiment
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

 Leave your comment on this article:
Leave your comment on this article: