
|
|
HISTORY OF TECHNOLOGY, TECHNOLOGY, OBJECTS AROUND US
Dynamite. History of invention and production
Directory / The history of technology, technology, objects around us Dynamite is an explosive mixture, an absorbent (for example, kieselguhr) impregnated with nitroglycerin. It may also contain other components (saltpeter, etc.). The whole mass is usually pressed into a cylindrical shape and placed in paper or plastic packaging. Undermining the charge is carried out using a detonator cap. Dynamite was patented by Alfred Nobel on November 25, 1867.
For several centuries, only one explosive was known to people - black powder, which was widely used both in war and in peaceful blasting. But the second half of the XNUMXth century was marked by the invention of a whole family of new explosives, the destructive power of which was hundreds and thousands of times greater than that of gunpowder. Their creation was preceded by several discoveries. As early as 1838, Peluz conducted the first experiments on the nitration of organic substances. The essence of this reaction lies in the fact that many carbonaceous substances, when treated with a mixture of concentrated nitric and sulfuric acids, give up their hydrogen, take in exchange the nitro group NO2 and turn into powerful explosives. Other chemists have investigated this interesting phenomenon. In particular, Shenbein, nitriding cotton, received pyroxylin in 1846. In 1847, acting in a similar way on glycerin, Sobrero discovered nitroglycerin, an explosive that had colossal destructive power. At first, nitroglycerin did not interest anyone. Sobrero himself returned to his experiments only 13 years later and described the exact method of glycerol nitration. After that, the new substance found some use in mining. Initially, it was poured into the well, plugged with clay and blasted by means of a cartridge immersed in it. However, the best effect was achieved by igniting a percussion cap with mercury fulminate. What explains the exceptional explosive power of nitroglycerin? It was found that during the explosion, it decomposes, as a result of which CO gases are first formed2,CO,H2, CH4, N2 and NO, which again interact with each other with the release of a huge amount of heat. The final reaction can be expressed by the formula: 2C3H5(NO3)3 = 6CO2 + 5H2O+3N+0,5O2. Heated to a huge temperature, these gases rapidly expand, exerting tremendous pressure on the environment. The end products of the explosion are completely harmless. All this seemed to make nitroglycerin indispensable in underground blasting. But it soon turned out that the manufacture, storage and transportation of this liquid explosive was fraught with many dangers. In general, pure nitroglycerin is quite difficult to ignite from an open flame. A lit match rotted in it without any consequences. But on the other hand, its sensitivity to shocks and concussions (detonation) was many times higher than that of black powder. Upon impact, often quite insignificant, in the layers subjected to shaking, there was a rapid increase in temperature until the explosive reaction began. The mini-explosion of the first layers produced a new impact on the deeper layers, and this continued until the explosion of the entire mass of matter occurred. Sometimes, without any external influence, nitroglycerin suddenly began to decompose into organic acids, quickly darkened, and then the most insignificant shaking of the bottle was enough to cause a terrible explosion. After a number of accidents, the use of nitroglycerin was almost universally banned. Those industrialists who set up the production of this explosive had two options left - either to find a condition in which nitroglycerin would be less sensitive to detonation, or to curtail their production. One of the first who became interested in nitroglycerin was the Swedish engineer Alfred Nobel, who founded a plant for its production. In 1864, his factory took off with the workers. Five people died, including Alfred's brother Emil, who was barely 20 years old. After this disaster, Nobel was threatened with significant losses - it was not easy to convince people to invest in such a dangerous enterprise. For several years he studied the properties of nitroglycerin and eventually managed to establish a completely safe production of it. But the problem of transportation remained. After many experiments, Nobel found that nitroglycerin dissolved in alcohol is less sensitive to detonation. However, this method did not provide complete reliability. The search continued, and then an unexpected incident helped to solve the problem brilliantly. When transporting bottles of nitroglycerin, in order to soften the shaking, they were placed in diatomaceous earth, a special diatomaceous earth mined in Hanover. Kieselguhr consisted of flint shells of algae with many cavities and tubules. And once, during the shipment, one bottle of nitroglycerin broke and its contents spilled onto the ground. Nobel had the idea to make some experiments with this diatomaceous earth impregnated with nitroglycerin. It turned out that the explosive properties of nitroglycerin did not decrease at all from the fact that it was absorbed by the porous earth, but its sensitivity to detonation decreased several times. In this state, it did not explode either from friction, or from a weak blow, or from burning. But on the other hand, when a small amount of mercury fulminate was ignited in a metal capsule, an explosion of the same force occurred that gave pure nitroglycerin in the same volume. In other words, it was exactly what was needed, and even much more than what Nobel hoped to get. In 1867, he took out a patent for a compound he discovered, which he called dynamite. The explosive power of dynamite is as huge as that of nitroglycerin: 1 kg of dynamite in 1/50000 of a second develops a force of 1000000 kgm, that is, sufficient to lift 1000000 kg per 1 m. Moreover, if 1 kg of black powder turned into gas for 0 seconds, then 01 kg of dynamite in 1 seconds. But with all this, well-made dynamite exploded only from a very strong blow. Ignited by the touch of fire, it gradually burned without an explosion, with a bluish flame. The explosion occurred only when a large mass of dynamite was ignited (more than 0 kg). Undermining dynamite, like nitroglycerin, was best done using detonation. For this purpose, Nobel in the same year 1867 invented a rattling primer detonator. Dynamite immediately found the widest application in the construction of highways, tunnels, canals, railways and other objects, which largely predetermined the rapid growth of the fortune of its inventor. Nobel founded the first factory for the production of dynamite in France, then he set up its production in Germany and England. For thirty years, the dynamite trade brought Nobel enormous wealth - about 35 million crowns.
The process of making dynamite was reduced to several operations. First of all, it was necessary to obtain nitroglycerin. This was the most difficult and dangerous moment in the entire production. The nitration reaction occurred when 1 part of glycerol was treated with 6 parts of concentrated nitric acid in the presence of XNUMX parts of concentrated sulfuric acid. The equation looked like this: C3H5(OH)3 +3HNO3 = C3H5(I have not3)3 + 3H2O. Sulfuric acid did not participate in the compound, but its presence was necessary, firstly, to absorb the water released as a result of the reaction, which otherwise, diluting nitric acid, would thereby prevent the completeness of the reaction, and, secondly, to isolate the resulting nitroglycerin from a solution in nitric acid, since it, being highly soluble in this acid, did not dissolve in its mixture with sulfuric acid. Nitration was accompanied by a strong release of heat. Moreover, if, due to heating, the temperature of the mixture rose above 50 degrees, then the course of the reaction would go in the other direction - the oxidation of nitroglycerin would begin, accompanied by a rapid release of nitrogen oxides and even greater heating, which would lead to an explosion. Therefore, nitration had to be carried out with constant cooling of the mixture of acids and glycerol, adding the latter little by little and constantly stirring each portion. Nitroglycerin formed directly in contact with acids, having a lower density compared to the acid mixture, floated to the surface, and it could be easily collected after the reaction was completed. The preparation of the acid mixture at the Nobel factories took place in large cylindrical cast-iron vessels, from where the mixture entered the so-called nitration apparatus.
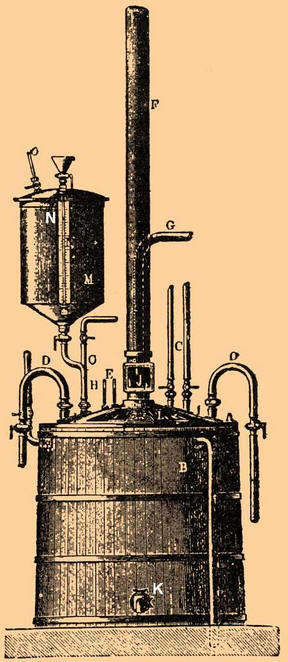
The apparatus consisted of a lead vessel A, which was placed in a wooden vat B and closed with a lead lid L, which was smeared with cement during operation. The ends of two lead coils D, located inside the apparatus, passed through the lid (cold water was constantly supplied through them). Cold air was also supplied to the apparatus through tube C to stir the mixture. Tube F removed nitric acid vapor from the apparatus; tube G served to pour a measured amount of the acidic mixture; glycerol was poured through tube H. In the vessel M, the required amount of this substance was measured, which was then injected into the nitrogen mixture by means of compressed air admitted through the tube O. In such an installation, about 150 kg of glycerol could be processed at a time. Having let in the required amount of the acid mixture and cooled it (by passing cold compressed air and cold water through the coils) to 15-20 degrees, they began to spray the cooled glycerin. At the same time, they made sure that the temperature in the apparatus did not rise above 30 degrees. If the temperature of the mixture began to rise rapidly and approached the critical, the contents of the vat could be quickly released into a large vessel of cold water. The operation of forming nitroglycerin lasted about an hour and a half. After that, the mixture entered the separator - a lead rectangular box with a conical bottom and two taps, one of which was located at the bottom and the other at the side. Once the mixture had settled and separated, the nitroglycerin was released through the top tap and the acid mixture through the bottom. The resulting nitroglycerin was washed several times to remove excess acids, since the acid could react with it and cause its decomposition, which inevitably led to an explosion. To avoid this, water was supplied to the hermetic vat with nitroglycerin and the mixture was mixed with compressed air. The acid dissolved in water, and since the densities of water and nitroglycerin differed greatly, it was not difficult to separate them from each other. In order to remove residual water, nitroglycerin was passed through several layers of felt and table salt. As a result of all these actions, an oily yellowish liquid was obtained, odorless and very poisonous (poisoning could occur both by inhalation of vapors and by contact of drops of nitroglycerin on the skin). When heated above 180 degrees, it exploded with terrible destructive force. The prepared nitroglycerin was mixed with diatomaceous earth. Before this, the diatomaceous earth was washed and thoroughly ground. Impregnation with nitroglycerin took place in wooden boxes lined with lead inside. After mixing with nitroglycerin, the dynamite was rubbed through a sieve and stuffed into parchment cartridges. In kieselguhr dynamite, only nitroglycerin was involved in the explosive reaction. Later, Nobel came up with the idea of impregnating various grades of gunpowder with nitroglycerin. In this case, gunpowder also participated in the reaction and significantly increased the force of the explosion. Author: Ryzhov K.V.
▪ Battery
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ PDA BrailleNote PK for the blind ▪ Mercedes will make trucks more economical
▪ section of the site Home workshop. Article selection ▪ article Wolf in sheep's clothing. Popular expression ▪ article How did the Golden Fleece end up in Colchis? Detailed answer ▪ Gambir article. Legends, cultivation, methods of application ▪ article Optoelectronic relay. Encyclopedia of radio electronics and electrical engineering
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese



 See other articles Section
See other articles Section 