
|
|
ENCYCLOPEDIA OF RADIO ELECTRONICS AND ELECTRICAL ENGINEERING Working with metals
Encyclopedia of radio electronics and electrical engineering / Ham Radio Technologies 1.1. Metal selection . When working with metals, their properties must be taken into account. low carbon steel is brazed and welded. They are used to make wire, mesh, welded structures, fasteners of medium strength. carbon steels with a carbon content of 0,5, as being hardened, are used for the manufacture of high-strength parts that work against abrasion. Instrumental steels can be subjected to all types of heat treatment. Steel grades U7 and U8 are suitable for the manufacture of hammers, chisels, screwdrivers, carpentry tools, saws for metal. Taps, dies, drills, files, scrapers, measuring tools are made from steel grades U12 and U13. Steel with a chromium content is used for the manufacture of turning tools, including those for hard materials. Steel containing manganese or silicon is used for the manufacture of cold springs, spring washers, etc. These steels can be subjected to all types of heat treatment. Copper - metal with low electrical resistivity. Used for winding wire, current-carrying parts of switches, etc. copper alloys (brass, bronze, etc.) are used for various crafts in amateur practice, for example, cores, decorative elements. Copper and its alloys are easily machined, nickel-plated, chrome-plated, silver-plated, and also painted in various original colors. Aluminum grades A1, A2, AZ has high plastic properties, which allows it to be used for capacitor plates, screens for loop coils, etc. Duralumin - an aluminum alloy with various components that increase strength, which makes it possible to make parts operating under load from it. A brand is affixed on sheet duralumin, the last letters of which denote: hot-rolled sheets - the letter A (D1A), annealed - the letter M (D1AM), hardened and naturally aged - the letter T (D1AT), etc. 1.2. Definition of steel grade quite accurately can be produced by a beam of sparks formed during processing on an emery wheel. The shape and length of the spark filaments, the color of the sparks, the shape of the beam are different for different steel grades: mild steel - continuous straw-yellow strands of sparks with a small number of stars at the ends of the strands; carbon steel (with a carbon content of about 0 5 ) - a bunch of light yellow threads of sparks with stars; tool steel U7 - U10 - a divergent bundle of light yellow threads with a large number of stars; tool steel U12, U13 - a dense and short bunch of sparks with a very large number of stars; asterisks are more "branched"; tool steel with a chromium content - a dense bunch of dark red threads of sparks and a large number of yellow stars; asterisks strongly branched; high speed steel with chromium and tungsten content - a bunch of discontinuous dark red strings of sparks, at the ends of which are lighter drop-shaped stars; silicon spring steel - a wide bunch of dark yellow sparks with lighter stars at the ends of the threads; high speed steel with cobalt content - a wide bunch of dark yellow threads of sparks without stars. 1.3. Heat treatment of metals and alloys, used in amateur practice, is divided into annealing, hardening and tempering. Annealing a steel part is produced to reduce its hardness, which is necessary to facilitate mechanical, including plastic, processing. Annealing is useful in cases where it is necessary to make a tool using the metal of another previously hardened tool. Complete annealing occurs when the part or workpiece is heated to 900C, held at this temperature to heat the part throughout its volume, and then slowly cooled to room temperature. The temperature of a hot part can be determined by the glow of the material:
Hardening gives the steel part greater hardness and wear resistance. The part is heated to a certain temperature, kept for some time necessary to warm up the entire volume of the material, and then quickly cooled. Typically, parts of structural steels are heated to 880 - 900, from tool to 750 - 760, from stainless steel - up to 1050 - 1100 C. A solution of common salt or oil is used for cooling. When cooled in oil, a dense film of oxides forms on the steel surface, which is a good anti-corrosion coating. When hardening small parts, you can easily overheat them. To avoid this, they use a method that has justified itself: they heat a large flat blank, on which a small part is placed. The temperature of the hardened part is determined by the color of the glow of the blank. It is necessary that during the cooling of the part the temperature of the liquid remains almost unchanged, so the mass of the liquid should be 30-50 times the mass of the hardened part. For intensive cooling, the part must be moved in all directions. Thin wide parts should not be immersed in a liquid, flat, as this will warp the part. Vacation hardened parts can reduce their brittleness to acceptable limits, while maintaining the hardness acquired by steel as a result of hardening. The heating temperature of a hardened steel part during tempering can be determined by the change in the color of the oxide film:
Below are the recommended tempering temperatures for some tools and parts (in degrees Celsius): Cutters made of carbon steels ....................................................... ......................180-200
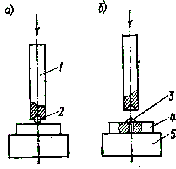
During hardening, duralumin parts are heated to 360 - 400C, kept for some time at this temperature, and then immersed in water at room temperature and left until completely cooled. After that, duralumin becomes soft and ductile, easily bent and forged. It acquires increased hardness after 3-4 days: its hardness and brittleness increase so much that it cannot withstand bending even at a small angle. During annealing, the part is heated to 360C, held for some time, and then cooled in air. To leave, the part is slightly heated and rubbed with laundry soap. Then continue heating until the layer of soap turns black, after which it is allowed to cool in air. (Blackening occurs at the tempering temperature.) Approximately, the heating temperature of a duralumin part can be determined as follows. At a temperature of 350 - 360C, the end of the match, free from sulfur, which is carried out over the hot surface of the part, is charred and leaves a dark mark. The temperature can be determined quite accurately using a small piece of copper foil (the size of a match head), which is placed on the surface of the heated part. At a temperature of 400C, a greenish flame appears above the foil. Hardening of copper occurs when a preheated part is slowly cooled in air. For annealing, the heated part is quickly cooled in water. During annealing, copper is heated to red heat (600C), while quenching - up to 400C, determining the temperature also with a piece of copper foil. In order for brass to become soft, easily bent, forged and well drawn, it is annealed by heating to 500C and air at room temperature. 1.4. Rust removal metal surfaces are usually produced with steel brushes (card brushes) or sandpaper, but chemical agents are more effective, for example, Auto Rust Converter. When using it, the metal surface should be cleaned with a spatula from loose and seam rust, and then degreased with white spirit or gasoline. Then, after thoroughly stirring, the composition is applied to the surface with a brush. The interaction of the composition with rust is indicated by a change in the color of the surface - it becomes bluish-violet. Work should be in rubber gloves and goggles. In case of contact with skin, rinse immediately with water. Another remedy is the Auto Rust Cleaner paste. It is applied to a metal surface, previously cleaned of loose and sheet rust and degreased, in a layer 2–3 mm thick and kept for 30 minutes. This operation can be repeated several times until the metal is free from rust. Good results are obtained when cleaning with a composition that is prepared from two solutions. The first of them: 250 g of ammonium, 53,5 g of caustic soda (caustic soda), 52 g of 200% formalin are dissolved in 40 ml of water and another 250 ml of water are added. The second is a 10% solution of hydrochloric or sulfuric acid. To one liter of the second solution add 30 ml of the first, and the composition is ready. Before immersing the part in the composition, it is thoroughly degreased in gasoline and dried. In the composition, the part is left for 10 - 30 minutes until the oxides are completely dissolved. After processing, the part is washed with hot water and wiped dry. Rust can also be removed electrochemically. A small piece of zinc is attached to the rusty part and immersed with it in water slightly acidified with sulfuric acid. With good zinc contact with the part, the rust disappears after a few days. The cleaned part is washed in warm water and wiped with a cloth. It is good to clean the rusty surface with fish oil, leaving a layer of fat for 1,5-2 hours. After exposure, the rust is easily removed. It should be noted that fish oil, penetrating the entire depth of rust, forms a film under it that prevents further rusting of the part. If it is necessary to quickly remove rust, then first the part is washed for several minutes in a saturated solution of chlorine tin, and then in warm water and wiped dry. Small spots of rust can be removed with a swab dipped in kerosene, as well as a swab with porridge made from crushed charcoal mixed with engine oil. In the latter case, the part is not only cleaned, but also polished. Places cleaned of rust are wiped with fine hot sand or wood ash, and, if necessary, painted over. 1.5. Sheet metal dressing . Editing (straightening) the waviness of the strip or the edges of the sheet is carried out by hitting a mallet or a steel hammer with a smoothly polished convex striker (see also paragraph 5.39) - from the middle to the edges of the bulge. Stronger blows are applied in the middle and the force of the blow decreases as it approaches the edges. Editing of long narrow sickle-shaped blanks is carried out on a plate. The workpiece is placed on the plate, pressed with one hand and struck with a hammer, starting from the shorter (concave) edge. At the beginning of dressing, the impacts should be stronger, and then gradually weaken as they approach the opposite edge. Before starting to edit the convex places (bulges), they are outlined with chalk or a pencil, then the workpiece is placed on the plate with the bulge up and strikes are started in the direction from the edges of the bulge to its center. The blows are frequent, but not strong. As you approach the center, the blows should be weaker. You can not immediately hit the most convex place from this, it will increase even more in area. Strips made of soft aluminum and copper alloys are best straightened through a gasket made of getinaks or textolite with a thickness of 1,5-3 mm. In this case, a smooth, undamaged surface is obtained even when working with a conventional steel hammer. Thin (up to 0,5 mm) sheet metal is corrected on a steel plate using a metal or wooden bar with rounded edges. 1.6. Workpiece marking consists in transferring points and lines (marks) from a drawing or sample to the surface of the workpiece. To do this, it is enough to have: two steel measuring rulers 150 and 300 mm long, a scriber, a center punch, a small hammer weighing 100-200 g, an ordinary drawing compass, a bench square and a caliper with a depth gauge. The scriber is a piece (150-200 mm) of wire with a diameter of 3,5-4.5 mm made of steel U 10 or U 12. One end of its 20-30 mm long is hardened and sharply sharpened, and the other is bent into a ring with a diameter of 15-25 mm . For marking in hard-to-reach places, it is convenient to use a scriber in which the sharpened (working) end is bent at an angle of 90 and then hardened. The sharper the working part of the scriber, the greater the accuracy can be achieved when marking. It is better to draw the line once, i.e. for sure, since the second time is harder to hit. exactly the same place. If it is necessary to draw different lines, then it is advisable to first draw horizontal, then vertical and inclined lines, and only after that - arcs, roundings and circles. The surface condition of the marked material affects the accuracy of marking work. It must be cleaned of dirt, scale, rust. In order for the lines applied by the scriber to be clear, the surface of steel and cast iron blanks is painted with chalk before marking or covered with a solution of copper sulphate (copperized). When marking on soft. metals and alloys, such as duralumin, brass and others, use a well-sharpened hard pencil (2T, 3T). It is impossible to use steel scriber, since when drawing marks, the protective layer is destroyed and conditions are created for corrosion. The marking of sheet materials can be done as follows. Marking lines are pre-applied on a sheet of graph paper. This sheet is glued with a few drops of rubber glue to the workpiece, and through it, with a center punch, all the centers of the holes and the nodal points of the contour of the part are marked. After that, the graph paper is removed and the final marking and processing of the part is carried out. Marking a center hole at the end of a cylindrical part.
Rice. 1.1. Marking a center hole at the end of a cylindrical part. A simple way to mark a center hole at the end of a cylindrical part is illustrated in fig. 1.1. A rectangular piece of tin is bent at a right angle so that the width of its upper part is approximately equal to the radius of the cylinder (Fig. 1.1, a). The corner is pressed against the side surface of the part and four lines are drawn at the end at an angle of approximately 90 °. The center of the end of the part is inside a small space bounded by lines, and it can be marked with a center punch quite accurately (Fig. 1.1, b). Before drilling holes along the contour (if it is necessary to obtain a hole of large diameter or a curvilinear shape), it is required to mark the centers of the "contour" holes by punching. This time-consuming operation will be greatly simplified if you use a simple device: a center punch is equipped with a retractable pointed leg. Having set the required center-to-center distance with its help, they start punching, combining the tip of the leg with the previous marked center. 1.7. Workpiece bending it is made by bending it around some mandrel, the shape of which it takes, as well as in a vice or on a plate to the desired angle. Bending of thick workpieces is carried out by blows of a hammer, preferably a wooden one, which does not leave marks on the metal. In the process of bending, the so-called neutral layer remains unchanged in length, which for blanks symmetrical in cross section passes through the center of symmetry, and for asymmetrical ones through the center of gravity of the section. The inner layer is in compression, the outer layer is in tension. If the bending radius is very small, then cracks can form in the metal. To avoid this, you should not bend to a radius smaller than twice the thickness of the workpiece. Sheet metal after rolling has a fibrous structure. In order to avoid cracks, it should be bent across the fibers or so that the bending line makes an angle of more than 45 ° with the direction of rolling. To avoid fracture when bending sheet duralumin, the material is annealed along the bending line (clause 1.3). 1.8. Pipe bending, especially large diameter (30-40 mm), can be produced using a spring.
The spring can be made of steel wire on a special mandrel clamped into a drill chuck, which, in turn, is fixed in a vice. The mandrel is a steel bar of the appropriate diameter with a thread, a nut and a longitudinal groove at one end (which remains free when the bar is attached to a drill). The end of the spring wire is inserted into the groove and clamped with a nut, after which, by rotating the drill chuck, the spring is wound. To create the necessary tension, the wire is passed between two tightly compressed wooden planks. Having finished winding, the nut is unscrewed and the spring is removed from the mandrel. The same mandrel can be used to wind springs of a larger diameter, if you first wind metal foil or thick paper around it in several layers. A neat pipe bend can be obtained in other ways. 1. At one end, the pipe is closed with a metal plug, and molten lead or tin-lead solder is poured into the other. (To avoid burns, the pipe must first be dried well.) After bending, lead (solder) is smelted by heating the pipe with a blowtorch.
1.9. Hole drilling . With a large number of holes of different diameters, it is recommended to first drill them all with a drill, the diameter of which is equal to the diameter of the smallest hole, and only then drill the remaining holes to the desired size. To avoid errors, identical holes are marked. It should be borne in mind that holes whose diameter is only 1,2-1,5 times the diameter of the smallest hole are drilled immediately with a drill of the required size. Countersinking holes is done to give them a finished look. Countersinking is performed to a shallow depth (0,2-0,3 mm) on both sides with a special tool (counterborer) or a drill, the diameter of which is approximately twice the diameter of the hole. The drill is sharpened at an angle of 90 °. When drilling holes in steel, aluminum and its alloys, coolants must be used: for mild steels, technical vaseline; for hard aluminum alloy (type D16T) - laundry or toilet soap; for aluminum, organic glass, getinaks - soapy water. 1.10. Klepka used for permanent connection of parts. Rivets are usually made from steel, copper, brass, aluminum and other metals and alloys that can be forged. The length of the rivet rod is taken based on the total thickness of the parts to be riveted and the protruding part of the rod necessary to form the closing head. To form a flat (secret) head, the protruding end should be equal to half the diameter of the rod, and a semicircular head - one and a half diameters. The diameter of the rivet rod is chosen depending on the thickness of the sheets or parts to be riveted: d=2S, where 5 is the smallest thickness of the parts (sheets) to be riveted. The diameter of the holes for the rivets is made 0,1-0,2 mm larger than the diameter of the rivet rod, and the protruding end of the rod is slightly conical. This makes it easier to insert rivets into the holes.
Rice. 1.2. Making a crimp (a) and forming a rivet head (b) using a fixture With the help of a stretch (a steel rod with a recess-hole in the end, and the diameter and depth of the hole is slightly larger than that of the protruding part of the rivet), hitting it with a hammer, the riveted parts are tightly compressed. Then the rivet rod is riveted, trying to keep the number of blows to a minimum. To do this, first, the rod is upset with strong blows, then the head is formed with light blows of the hammer, and finally it is formed by crimping (the rod with a magnifying glass at the end in the shape of the rivet head). If you immediately install a crimp on the protruding end of the rivet and hit it, simultaneously riveting and shaping the head, then the head may be displaced relative to the axis of the rivet, which is undesirable. Rivets can be made yourself from copper or aluminum wire using a simple device shown in Fig. 1.2. It is a steel plate with a hole, the diameter of which is equal to the diameter of the wire. The thickness of the plate should be equal to the length of the rivet. For rivets with a semicircular head, the length of the workpiece should be 1,3-1,5 diameters greater than the length of the rivet. Plate 4 is placed on steel plate 5, workpiece 3 is inserted into the hole in the plate, and the protruding part of the workpiece is riveted with light blows of the hammer, trying to give it a shape close to hemispherical. The final molding of the rivet head is carried out using crimping 1. The finished rivet is knocked out of the plate from the reverse side with a steel rod, the diameter of which is 0,1-0,2 mm less than the diameter of the hole. The crimp is made from a steel or brass bar of a suitable diameter. A recess is made at the end of the bar with a drill, the diameter of which is approximately twice the diameter of the rivet. Then a steel ball 2 of the same bottom-meter drill is placed on the steel plate, a crimp is placed on it (a recess to the ball) and the recess is given a hemispherical shape by hammer blows on the free end of the crimp. If it is necessary to make rivets with a countersunk head, then the hole in the plate is countersinked on one side with a drill sharpened at an angle of 90 °. In this case, the length of the wire blank should be greater than the length of the rivet by 0,6-0,8 of the diameter. 1.11. Thread in holes cut with taps. For each standard thread size included. as a rule, two taps are included: the first is marked with one ring risk, the second with the letter E. The thread is cut first with the first tap, then with the second. To chip off the chips, the tap after each turn clockwise is turned half a turn in the opposite direction. During operation, the taps are fixed in special holders (knobs). Convenient For threads smaller than M4, use handles ("beaks") from switches for this purpose. To improve the quality of the thread, it is recommended to use the same cutting fluids as for drilling. The diameter of the threaded hole is approximately determined by multiplying the thread size by 0,8 (for example, for an M2 thread, the drill should have a diameter of 1,6 mm, for MZ-2,4 mm, for M4-3,2 mm, etc.) . For the reliability of the threaded connection, the thread size is chosen so that there are at least three full threads of thread in the threaded hole. So, with a material thickness of 2 mm, it is necessary to cut the M2 and M0,4 threads, in which the pitch is 0,5 and 4 mm, respectively. It is not advisable to use the M0,7 thread, since its pitch is XNUMX mm. When threading in blind holes, in order not to break the tap, after every two or three full turns, it should be unscrewed and the chips removed. In this case, it is useful to control the depth of the hole and the position of the tap in order to prevent its breakage. 1.12. External thread on bars it is cut into dies fixed in die holders. To obtain a clean thread, the bar diameter must be slightly smaller than the thread size. Before cutting, the processed part of the bar is lubricated with machine oil or technical petroleum jelly. To break off the chips, after each clockwise revolution, the die is turned half a turn in the opposite direction. 1.13. Cleaning contaminated surfaces details from aluminum alloys is made by etching. To do this, the part is treated for 1-2 minutes in a 5% sodium hydroxide solution, washed in water, dipped in nitric acid and washed again. After that, the metal acquires a pure silver color. The appearance of duralumin parts will be significantly improved if their surfaces are lubricated with an aqueous solution of borax (1 g of borax per 100 ml of boiled water) with the addition of a few drops of ammonia. After 30 minutes, the parts are wiped with a clean cloth rag. The surfaces of copper, brass and bronze parts are cleaned with a paste consisting of equal parts of talc and sawdust mixed with table vinegar until a pasty mass is obtained. Good results are obtained when using a paste composed of equal parts of table salt and chalk mixed with whey. 1.14. Phosphating steel parts provides the formation of a protective film on the metal surface with high anti-corrosion properties. The cleaned, polished, degreased (for example, with gasoline) and pickled (for 1 min in a 5% sulfuric acid solution) steel part is immersed in a hot solution (35 g / l) of majef-phosphate salts of manganese and iron. The temperature of the solution should be 97-99 °C. In this case, a violent chemical process is observed with the release of a large amount of hydrogen. After an hour and a half, hydrogen evolution stops, the part is kept in solution for another 10-15 minutes, after which it is thoroughly washed with hot water, dried and lubricated with oil (Vaseline). 1.15. Oxidation of steel (iron) is a kind of anti-corrosion and decorative coating. Among such methods as phosphating, chemical nickel plating, oxidation, the latter is the simplest, not labor-intensive, and does not require special expenses. The cleaned, polished part is decapitated (dipped in a 1% sulfuric acid solution for 5 minute), then washed in water at room temperature and passivated by boiling for about 5 minutes in soapy water (50 g of laundry soap is dissolved in a liter of water). After that, a solution of caustic soda (50 g / l) is prepared in enameled dishes, heated to 140 ° C and the part is immersed in it for 1,5 hours. As a result, a shiny black film forms on the metal surface. If you need a matte black film, then dissolve 50 g of sodium nitrate and 1500 g of caustic soda in one liter of water, heat the solution to 150 ° C and immerse the part in it for 10 minutes. 1.16. bluing gives a good appearance to steel parts. In this case, the part is covered with an oxide film that prevents metal corrosion and has a pleasant tone - from blue to black. Before bluing, the part is carefully ground and polished, then it is degreased by wiping it with a swab dipped in gasoline. For degreasing, you can use an aqueous solution of washing powder. After that, the part is heated to a temperature of 250-300 ° C and wiped with a swab soaked in hemp oil. To increase the anti-corrosion properties, the cooled part is wiped with technical petroleum jelly, then wiped dry. There is another way of bluing; the fat-free part is immersed in molten sodium nitrate (310-350 ° C). Within 3-5 minutes, a thin but very strong film of a beautiful bluish tint forms on the surface of the immersed part. 1.17. Anodizing of aluminum and aluminum alloys. The process ensures the formation of a stable protective film that can be dyed in any color. When anodizing with direct current, the part is first polished to a mirror finish (there should be no scratches or dents), degreased with acetone and then for 3-5 minutes with a solution of caustic soda (50 g / l). The temperature of the solution should be around 50°C. After degreasing, it is desirable to carry out chemical polishing. To do this, the part must be placed for 5-10 minutes in a composition of 75 volume parts of ortho-phosphoric acid and 25 sulfuric acid. The temperature of the composition should be 90-100 °C. After polishing, the part is washed and lowered into a bath filled with a 20% sulfuric acid solution (electrolyte temperature is not more than 20°C). The bathroom can be glass, ceramic or enamelware. The hanger for the part must be aluminum. The anode is a detail. The cathode is a lead plate. Contacts of conductors (aluminum) with the anode and cathode must be very reliable, best done by riveting or soldering. The voltage on the electrodes is maintained at 10-15 V. The anode current density for aluminum parts is 0,15-0,20, for duralumin parts 2-3 A / dm. The required current density can be provided by changing the voltage within the specified limits and changing the distance between the electrodes. Anodizing time 25-50 min. The quality of anodizing is checked as follows. With a chemical pencil, draw a line along the anodized surface of the part (in an inconspicuous place). If the dash won't wash off with running water, the anodizing is done well. After checking, the part is washed and immersed in an aqueous solution of aniline dye for 10-15 minutes. The temperature of the solution is 50-60 °C. If the part is dipped in a 10% solution of potassium dichromate (chromic) for 10-12 minutes at 90 ° C, then it will turn golden. The final process is sealing (closing) of film pores. The pores are compacted after boiling the part in water for 15-20 minutes. After drying, the part can be coated with a colorless varnish or glue BF-2, BF-4. When anodizing with alternating current, all preparatory and final operations are similar to those described above. The peculiarity is that two parts are anodized at once (if there is one part, then an aluminum sheet or blank is used as the second electrode). With an alternating voltage of 10-12 V, the same current density is achieved as during anodizing with direct current. Anodizing time 25-30 min. 1.18. Oxidation of aluminum and aluminum alloys provides protection of details from corrosion.
For oxidation, a solution is prepared containing 50 g of soda ash, 15 g of sodium chromate and 1 g of sodium silicate per liter of distilled (in extreme cases, boiled) water. In a solution heated to 80 ° C, the part is lowered for 10 minutes. Then it is thoroughly washed in running water. It is possible to propose another method for the oxidation of aluminum. The part is brushed (they clean the surface with a card brush), making small strokes in different directions, creating a certain pattern. Chips and dirt are removed with a clean rag. Then the surface of the part is covered with an even layer of 10% sodium hydroxide solution (solution temperature 90-100 ° C). After the solution dries, a beautiful film with a pearly sheen forms on the surface of the part. From above the film is covered with a colorless varnish. The film will turn out to be more beautiful if the part is heated to 80-90 ° C before applying the caustic soda solution. 1.19. Painting of oxidized parts made of aluminum and aluminum alloys in different colors are produced by sequential chemical treatment in two 1% aqueous solutions of metal salts (Table 1.1). For dyeing black, the oxidized part is alternately treated in solutions of the following composition: 1st solution - 50 g / l of iron ammonium oxalate (solution temperature 60 ° C, exposure of 1 part 0,5-1 min); 2nd solution - 50 g/l cobalt acetate (50°C, 1-3 min) 3rd solution-50 g/l potassium permanganate (80°C, 3-5 min) Before treatment in each next solution, the part is washed in water. A golden-green color can be given to a part if it is treated for 2-4 minutes in a solution heated to 100 ° C with the following composition: 15 g of potassium dichromate and 4 g of soda ash per 1 liter of water. Table 1.1. Solutions for chemical painting of parts made of aluminum and aluminum alloys
1.20. Chemical nickel plating parts made of steel, copper and copper alloys can be performed in one of the following ways. The surface of the part is ground, polished, and then degreased. For degreasing steel parts, an aqueous solution of the following composition is used: caustic soda or caustic potash - 20-30, soda ash-25-50, liquid glass (silicate glue) -5-10 g / l. Aqueous solution for degreasing copper and copper alloys: trisodium phosphate - 100, liquid glass - 10-20 g / l. Degreasing in a room temperature solution lasts 40-60 minutes. When the solution is heated to 75-85 C, the process is significantly accelerated. The degreased part is thoroughly washed in running water and immersed in a 5% hydrochloric acid solution for 0,5-1 min for decapitation. The temperature of the solution should not exceed 20 °C. Then the part is thoroughly washed and immediately transferred to a nickel-plating solution (in air, the part is quickly covered with an oxide film). Nickel plating solution is prepared as follows. In a liter of water heated to 60 ° C, dissolve 30 g of nickel chloride and 10 g of sodium acetate. The temperature of the solution is brought to 80 ° C, 15 g of sodium hyposulfite are added and the part is immersed in the solution. The solution with the detail is heated to a temperature of 90-95 ° C, which is maintained until the end of nickel plating. At temperatures below 90 °C, the nickel plating process proceeds slowly, and when heated above 95 °C, the solution deteriorates. The volume of the solution in liters should be numerically equal to one third of the area of the part in square decimeters. The film growth rate is approximately 10 µm/h. Another method allows nickel-plating of copper, brass and bronze parts, provides a dense shiny film with good anti-corrosion properties. The method does not require complex equipment and special costs for materials. The part is cleaned and polished. Degrease in the solution, the recipe of which is given above. It is not necessary to decapitate. A 10% solution of zinc chloride ("soldering acid") is poured into enameled dishes and nickel sulfate is added to it until the solution becomes a deep green color. The resulting solution is heated to a boil and the part is lowered into it. The part should be in the boiling solution for 1-2 hours, then her transferred to chalk water (10-15 g of chalk per glass of water) and lightly wiped with a rag. Next, the part is washed and wiped dry with a rag. For repeated use, the solution can be stored for 6 months. in a tightly sealed container. Chemical nickel plating of aluminum almost does not differ from chemical nickel plating of steel, except that pickling is carried out by immersing the part for 2-3 minutes in a 50% solution of nitric acid. 1.21. Coloring of steel (iron). In order for the coating to be durable, the metal is carefully cleaned and primed, and each type of paint must correspond to a certain type of soil. When cleaning, parts are immersed in kerosene for a long time, then rust is removed from them and degreased. Rust can be removed in other ways (section 1.4). A feature of the soil is increased adhesion (the ability to adhere to the surface of the part). This ensures the strength of the entire coating (primer plus paint). The primer is placed on the surface of the part with a layer of no more than 0,2 mm thick and, after drying, it is cleaned with an emery cloth until it is completely leveled. As a kind of primer, vinegar essence can be used, which is used to wipe a well-cleaned and degreased part. Most paints, varnishes and enamels fit well on such a "primer". Paint the details with a soft brush in at least two layers. Moreover, each next layer is applied in a direction perpendicular to the previous one. It is convenient to paint with a sprayer, taking precautions to protect the fresh coating from clogging. In this case, nitroenamels, synthetic melamine-alkyd and alkyd enamels can be used. Nitro enamels dry quickly even at room temperature, but are very sensitive to moisture: when the relative humidity of the air is above 70%, the paint film may become covered with white spots when it dries. After drying, a semi-gloss finish is formed, the gloss of which can be increased to the desired degree by grinding and polishing. The processes of polishing and grinding are long and laborious. The adhesion of nitroenamels to metal is low, therefore, prior priming is necessary before painting. Nitroenamels are "reversible". This means that it is impossible to apply a second layer of nitro enamel with a brush without the risk of dissolving the previously applied layer. Synthetic melamine alkyd enamels form a durable glossy film. At a temperature of 100-130 ° C (depending on the type of enamel), freshly applied (the film dries in 30 minutes. Above 130 ° C, the enamel cannot be heated. At room temperature, such enamel, unfortunately, does not dry at all. It is impossible to grind dried enamel. It is polished with compounds containing wax.Adhesion to metal is good, therefore it can be painted without a primer. Alkyd enamels are close in nature to oil paints. They are similar in strength to synthetic melamine alkyd enamels and also react to grinding and polishing. Unlike synthetic enamels, they dry at room temperature in 2 days (if the temperature rises, this time can be significantly reduced). Some enamels are available in aerosol packaging. Steel balls are placed in cylinders with enamel. Their purpose is to help evenly mix the enamel and solvent contained in the balloon. Therefore, before use, it is necessary to shake the balloon until the sounds of balls hitting the walls of the balloon are heard. Moreover, shaking should be continued after that for another two to three minutes and only then proceed to staining. As a precaution, the jet is directed somewhere to the side, and only then, making sure that the enamel is evenly supplied, onto the surface to be painted. Table 1.2. Compositions (%) of washes and pastes for removing enamels and varnishes based on nitrocellulose, glyphthalic and nitrolithic resins
During the entire staining process, you need to make continuous uniform movements with a hand with a balloon, holding it at a distance of 25-30 cm from the surface. The paint jet must be perpendicular to the surface. During a break in work, it is necessary to blow out the cylinder valve, otherwise the enamel in the valve will dry out and it will become clogged. To do this, you need to turn the balloon over and press the start button: as soon as the jet coming out of the nozzle becomes colorless (the paint stops flowing), blowing should be stopped. 1.22. Removing old paintwork from metal products is carried out using washes and washing pastes (Table 1.2). A wash or paste is applied to the coating to be removed. After a while, the coating softens and can be easily removed. The presence of paraffin (wax) makes the composition thicker or even pasty. It is more convenient to work with a pasty composition than with a wash, which has to be applied to the surface to be treated several times. Did you know? 1.23 If you countersink with a hand drill a hole for a countersunk screw head in a viscous sheet metal (copper, aluminum, soft duralumin) with a thickness less than a third of the diameter of the drill and at the same time fix the part with clamps on a PCB or hard wood plate, then the conical recess will turn out more accurate. 1.24 You can flare a metal tube using a conventional drill, rotating it in the opposite (with respect to the working rotation) direction. In this case, the diameter of the drill should be 1,5-2 times larger than the diameter of the tube. 1.25 Instead of a rivet, you can use a liquid metal or an alloy that increases in volume during crystallization (gallium, germanium, tin, bismuth and their alloys). 1.26 In order for the thread cut with a tap in a blind hole to be clean, the hole must first be filled with molten paraffin. 1.27 When cutting threads in soft metals, such as aluminum, it is better to limit yourself to the first tap (1.11). In such a hole, the screw is held more firmly. 1.28 A thread broken after cutting a screw or stud can be easily restored if you first screw a die or nut onto them. Having cut off or bitten off the excess with wire cutters, the end of the threaded part is sawn off with a file, and then the die (nut) is screwed - the thread is restored. 1.29 You can wash dishes from kerosene with milk of lime: pour a little slaked lime into the vessel to be cleaned and, shaking often, fill it to the top with water. After a few hours, the contents are poured out, the vessel is rinsed with water and the procedure is repeated. Cleaning will be faster if coarse sand is added to the dishes. 1.30 After working with kerosene, solvents, paint, hands have a specific smell, and the best way to get rid of it is to wash your hands with mustard water or mustard powder. 1.31 It is more convenient to drill small washers and bushings by gently clamping them in the drill chuck; in this case, the drill is clamped in a vice. In thin-walled tubes, drilling holes is facilitated if a wooden rod is first placed inside the tube. 1.32 In the notch of the file, particles of the processed metal will not get stuck if the file is first rubbed with chalk or charcoal. 1.33 Rust is easily removed mechanically after surface treatment of the part with a saturated paraffin solution. In a vessel with kerosene, paraffin shavings are dissolved until saturation. The solution is ready in a week. The part is lubricated with a solution and left for several days. 1.34 Before soldering ferrous metal products, heavily rusted parts should be immersed for 12 hours in a saturated solution of zinc and hydrochloric acid (zinc chloride), diluted by half with distilled water. 1.35 Parts made of hard metals are best processed with files with a cross cut, soft metals - with a simple (single) notch. 1.36 Chassis made of aluminum or its alloys can be given a slightly matte finish by treating them in a 5% sodium hydroxide solution for 5 minutes. Beforehand, the chassis is carefully cleaned with fine-grained sandpaper and washed in soapy water. 1.37 You can freshen up aluminum chassis, panels and screens by washing them with a stiff bristle brush in a warm water solution of laundry soap. 1.38 You can give parts made of iron or steel black with a mixture of 10 parts of turpentine and 1 part of sulfur (finely ground sulfur). The components are mixed in a glass dish and heated in a water bath to a boil. The part is dipped into the mixture for 5-10 minutes. The blue color of a steel or iron part can be given by using a mixture of 4 parts of copper sulfate, 6 parts of nitric acid, 12 parts of ethyl alcohol and 100 parts of water. The mixture is prepared in glassware, not heated. The part is kept in the mixture until a blue color appears. Author: tolik777 (aka Viper); Publication: cxem.net
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ Salt ban ▪ Cultivation of GM tomatoes approved for cancer, diabetes and dementia
▪ site section Electrician's tool. Article selection ▪ article But only the strong in soul can be carried there by the waves. Popular expression ▪ article Why did Alcmene give birth to twins? Detailed answer ▪ article Lathe with manual drive. home workshop ▪ article Car radio watchman. Encyclopedia of radio electronics and electrical engineering
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

 Leave your comment on this article:
Leave your comment on this article: