
|
|
Lecture notes, cheat sheets
Materials Science. Lecture notes: briefly, the most important
Directory / Lecture notes, cheat sheets Table of contents
LECTURE No. 1. The structure of wood 1. Types of tree species and parts of a tree Growing trees have the following components: roots, trunk, branches, leaves. The root system of trees acts as a supplier of moisture and nutrients from the soil through the trunk and branches to the leaves. In addition, the roots hold the trees upright. Through the branches, moisture enters the leaves, in which the process of photosynthesis takes place - the conversion of the radiant energy of the sun into the energy of chemical bonds of organic substances with the absorption of carbon dioxide from the air and the release of oxygen. It is no coincidence that forests are called the lungs of the planet. The products of photosynthesis from the leaves are transmitted through the branches to the rest of the trees - the trunk and roots. Thus, the branches act as channels through which the exchange of substances takes place between the leaves and the rest of the tree. Coniferous trees - pine, cedar, spruce, larch - have narrow leaves - needles, and hardwoods - wide leaves. As a rule, deciduous trees grow mainly in temperate and southern latitudes, while conifers grow in northern ones. Depending on the species and climatic conditions of growth, trees have different heights and trunk diameters. However, they fall into three categories. The first includes trees of the first magnitude, which reach a height of 20 m or more. These are spruce, cedar, larch, pine, birch, aspen, linden, oak, ash, maple, etc. In the tropics and subtropics, the height of individual trees reaches 100 m or more. The second category includes trees of the second magnitude, having a height of 10-20 m. These are, in particular, willow, alder, mountain ash, etc. The third category is trees of the third magnitude, the height of which is 7-10 m. These are apple, cherry, juniper, etc. . The diameter of the tree trunk varies mainly from 6 to 100 cm or more and depends on the species, age of the trees and climatic conditions of growth. In some cases, the diameter of a tree trunk can exceed 3 m - in oak, poplar and some other species. Wood is obtained by cutting tree trunks after removing branches. In this case, the yield of wood is 90 or more percent of the volume of the tree trunk. At the initial stage of wood processing, a transverse, or end, section of the trunk is made. On the cross section, the following are distinguished: the bark covering the trunk from the outside and consisting of the outer layer - the crust and the inner layer - the bast cambium - a thin layer invisible to the eye between the bark and the wood (during the growth of trees, the living cells of the cambium divide, and due to this the tree grows in thickness); sapwood - living zone of wood; the core, which is adjacent to the core of the trunk and is a dead central zone that does not participate in physiological processes; the core, located in the center and representing a loose tissue with a diameter of 2-5 mm or more (depending on the species and age of the tree). In the timber industry in Russia, the main object of harvesting is tree trunks, and branches and branches are burned or used for firewood. In Canada, Sweden and Finland, all components of trees are recycled, so the loss of wood there is minimal, and the yield of paper, cardboard and other things is maximum. 2. Macroscopic structure of wood With a cross section of a tree trunk, you can establish the main macroscopic features: sapwood, heartwood, annual layers, medullary rays, vessels, resin canals and medullary repetitions. In young trees of all species, wood consists only of sapwood. Then, as they grow, the living elements around the core die off, and the moisture-conducting paths become clogged, and extractive substances gradually accumulate in them - resins, tannins, dyes. Some trees - pine, oak, apple and others - the central zone of the trunk acquires a dark color. Such trees are called sound. In other trees, the color of the central zone and sapwood of the trunk is the same. They're called non-core. Kernelless trees are divided into two groups: ripe-woody (linden, fir, beech, spruce), in which the humidity in the central part of the trunk is less than in the peripheral, and sapwood, in which the moisture content is the same across the cross section of the trunk (birch, maple, chestnut, etc.). Moreover, the mass of sapwood decreases from the top to the butt, as well as with an increase in the age of the tree. The age of trees can be determined by the number of annual layers that grow one per year. These layers are clearly visible on the cross section of the trunk. They are concentric layers around the core. Moreover, each annual ring consists of an inner and outer layer. The inner layer is formed in spring and early summer. It is called early wood. The outer layer is formed by the end of summer. Early wood has a lower density than late wood and is lighter in color. The width of the annual layers depends on a number of reasons: firstly, on the weather conditions during the growing season; secondly, on the growing conditions of the tree; thirdly, from the breed. On a cross section of trees, you can see the core rays extending from the center of the trunk to the bark. In hardwoods, they occupy up to 15% of the volume of wood, in conifers - 5-6%, and the greater their number, the worse the mechanical properties of wood. The width of the core rays ranges from 0,005 to 1,0 mm, depending on the tree species. Softwood wood differs from hardwood wood in that it contains cells that produce and store resin. These cells are grouped into horizontal and vertical resin ducts. The length of the vertical passages ranges from 10-80 cm with a diameter of about 0,1 mm, and the horizontal resin passages are thinner, but there are a lot of them - up to 300 pieces per 1 cm 2. Hardwood has vessels in the form of a system of cells for the transfer of water and minerals dissolved in it from the roots to the leaves. Vessels are in the form of tubes with an average length of 10 cm and a diameter of 0,02-0,5 mm, and in trees of some species they are concentrated in the early zones of the annual layers. They are called annular. In trees of other species, the vessels are distributed over all annual layers. These trees are called diffuse-vascular. 3. Microscopic structure of coniferous and hardwood wood Coniferous wood has a certain microstructure, which can be established using microscopes, as well as chemical and physical research methods. Coniferous wood differs from hardwood in a relatively regular structure and simplicity. The structure of coniferous wood includes the so-called early and late tracheids. As established by research, early tracheids function as conductors of water with minerals dissolved in it, which comes from the roots of the tree. Tracheids are in the form of strongly elongated fibers with oblique ends. Studies have shown that in a growing tree, only the last annual layer contains living tracheids, and the rest are dead elements. As a result of the research, it was revealed that the core rays are formed by parenchymal cells, along which reserve nutrients and their solutions move across the trunk. The same parenchymal cells are involved in the formation of vertical and horizontal resin ducts. Vertical resin canals in coniferous wood, found in the late zone of the annual layer, are formed by three layers of living and dead cells. Horizontal resin ducts were found in the medullary rays. According to the research results of Professor V. E. Vikhrov, pine wood has the following microscopic structure: 1) cross section; 2) radial incision; 3) tangential cut.  Rice. 1. Sections of a tree trunk: P - transverse, R - radial, T - tangential As established by research, the microstructure of hardwood compared to coniferous wood has a more complex structure. In hardwood, vascular and fibrous tracheids serve as conductors of water with minerals dissolved in it. The same function is performed by other vessels of wood. The mechanical function is performed by libriform fibers and fibrous tracheids. These vessels are in the form of long vertical tubes, consisting of separate cells with wide cavities and thin walls, and the vessels occupy from 12 to 55% of the total volume of hardwood. The largest part of the volume of hardwood is made up of libriform fibers as the main mechanical fabric. Libriform fibers are elongated cells with pointed ends, narrow cavities and powerful walls with slit-like pores. Fibrous tracheids, like libriform fibers, have thick walls and small cavities. In addition, it was found that the core rays of deciduous wood unite the main part of parenchymal cells, and the volume of these rays can reach 28-32% (this figure applies to oak). 4. Chemical composition of wood The chemical composition of wood depends partly on its condition. The wood of freshly cut trees contains a lot of water. But in a completely dry state, wood consists of organic substances, and the inorganic part is only from 0,2 to 1,7%. During the combustion of wood, the inorganic part remains in the form of ash, which contains potassium, sodium, magnesium, calcium and, in small quantities, phosphorus and other elements. The organic part of wood of all species has approximately the same elemental composition. Absolutely dry wood contains on average 49-50% carbon, 43-44% oxygen, about 6% hydrogen and 0,1-0,3% nitrogen. Lignin, cellulose, hemicellulose, extractive substances - resin, gum, fats, tannins, pectins and others - make up the organic part of wood. Hemicellulose contains pentosans and genxosans. Coniferous species have more cellulose in the organic part, while deciduous species have more pentosans. Cellulose is the main component of the cell walls of plants, and it also provides the mechanical strength and elasticity of plant tissues. As a chemical compound, cellulose is a polyhydric alcohol. When cellulose is treated with acids, it is hydrolyzed with the formation of ethers and esters, which are used for the production of films, varnishes, plastics, etc. In addition, during the hydrolysis of cellulose, sugars are formed, from which ethyl alcohol is obtained by fermentation. Wood cellulose is a valuable raw material for paper production Another component of the organic part of wood - hemi-cellulose - is a polysaccharide of higher plants, which are part of the cell wall. In the process of processing cellulose, lignin is obtained - an amorphous polymeric substance of a yellow-brown color. The largest amount of lignin - up to 50% - is formed during the processing of coniferous wood, and its yield from hardwood is 20-30%. Very valuable products are obtained during the pyrolysis of wood - dry distillation without air at temperatures up to 550 ° C - charcoal, liquid and gaseous products. Charcoal is used in the smelting of non-ferrous metals, in the production of electrodes, medicine, as a sorbent for sewage treatment, industrial waste, and for other purposes. Such valuable products as gasoline antioxidant, antiseptics - creosote, phenols for the production of plastics, etc. are obtained from the liquid. In the organic part of coniferous wood there are resins that contain terpenes and resin acids. Terpenes are the main raw material for the production of turpentine. The resin secreted by the coniferous tree serves as a raw material for the production of rosin. In the process of processing wood, extractive substances are obtained, including tannins, used for dressing leather - tanning. The main part of tannins are tannins - derivatives of polyhydric phenols, which, when processed, interact with their protein substances and form insoluble compounds. As a result, the skins acquire elasticity, resistance to decay and do not swell in water. LECTURE No. 2. Types of wood defects 1. Knots, cracks Defects of wood - these are deviations from the norm in the structure of the trunk, all violations of the physical condition. The defects include: knots, cracks, defects in the shape of the trunk, wood structure, chemical stains, fungal infections, biological and mechanical damage, processing defects and warping. The most common vice is bitches - the bases of the branches that are present in the wood of the trunk. When cutting wood, knots of various shapes and types are revealed on its surface. According to the shape of the cut on the surface of the wood, you can see round, oval and oblong knots, and according to the degree of intergrowth with the wood, they are also subdivided into intergrown, partially intergrown and not intergrown, or falling out. When cutting wood into boards, knots can have a different position - plastic, edge, rib, stitched - in the case of a longitudinal section of a knot, part of it goes simultaneously to two edges of the same side of the board and end - when the knot is at the end of the board. According to the mutual arrangement of knots on lumber, they are divided into scattered - solitary or separated from each other at a considerable distance, grouped and branched. As of wood of the knot body itself, they are divided into: light healthy, dark healthy, healthy with cracks, rotten, rotten and "tobacco", in which rotten wood is completely or partially replaced by a loose mass of rusty-brown or whitish color. The presence of knots in wood leads to a decrease in strength, makes it difficult to process and glue, reduces quality (especially with a large number and diameter of them). Unjoined and rotten knots significantly reduce the quality of wood, and in some cases they make the wood unsuitable for the manufacture of products (for example, boards). Another type of wood defect is cracks, formed when wood is torn along the grain. Cracks appear in a growing and felled tree. The first include metic, peeling and frosty, the second - shrinkage cracks. Metic cracks that pass through the core of a tree trunk have the greatest extent, and when the harvested wood dries, their dimensions increase. In round blanks of wood, such cracks usually occur at the ends, in lumber or parts - at the ends and side surfaces. When the wood is stratified along the annual layer, peeling cracks are formed, and usually at the border of a sharp transition from interlayer wood to large-layer wood, and are found in trees of all species. During the drying of the wood, the peeling crack increases. When drying wood under the influence of internal stresses, shrinkage cracks. This type of cracks differs from others (metic and frost cracks) in a smaller length and depth. In boards, cracks can go to the face, edge or end. Accordingly, they are called sheet, edge and end. Cracks, especially through cracks, violate the integrity of the wood material and reduce its mechanical strength. 2. Defects in the shape of the trunk The processing of wood of all species is very often complicated by the occurring defects in the shape of the trunk: tapering, ovality, outgrowths, curvature and stubble. Escape is expressed in a decrease in the diameter of a log or the width of an unedged board, exceeding the normal run-off, which is equal to 1 cm per 1 m of the length of the assortment. As a rule, it is greater in hardwoods, especially in trees grown in the open, and along the length of the trunk - in the top part. This type of trunk shape defect increases the amount of waste when sawing and peeling round timber and causes the appearance of a radial inclination of the fibers in the veneer. ovality the trunk is an elliptical shape of the cross section of the end, in which the larger diameter is 1,5 or more times greater than the smaller one. The growths in the form of a local thickening of the trunk of various shapes and sizes complicate the processing of wood. growths are formed as a result of tissue growth under the influence of various irritants - fungi, low or high temperatures, etc., as well as during fires, mechanical damage and for other reasons. 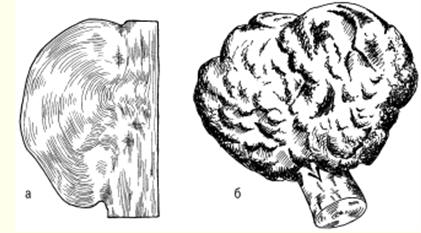 Rice. 2. Growths: a) smooth, b) bumpy Smooth outgrowths (Fig. 2a) often appear on pine and birch trunks. Annual layers in places of growths are usually wider than in the trunk. Hilly outgrowths, or burls (Fig. 2b), are formed mainly on the trunks of birch, walnut, as well as maple, black alder, ash, beech, poplar, etc. Wood in the burl zone has an irregular structure with a wavy-wavy direction of the fibers and with dark-colored inclusions in the form of small spots, dashes and dots. In cuts, the caps have a beautiful texture, so they are used as a material for art crafts and for the manufacture of sliced veneer. Such a defect of the trunk as his curvature, also makes it difficult to use roundwood and increases sawing waste. The curvature of the trunk is the deviation of the longitudinal axis from a straight line, and it can be with one bend and complex - with two or more bends. Often there is a type of malformation of the trunk, such as buttiness, which is expressed in a sharp increase in the diameter of the butt of round timber, i.e. when the diameter of the butt end is 1,2 times greater than the diameter at a distance of a meter from this end. When sawing and peeling wood, the presence of such a defect leads to an increase in the amount of waste and, in addition, causes the appearance of a radial inclination of the fibers in the veneer. The buttiness also complicates the use of roundwood for its intended purpose and complicates the processing of wood. 3. Defects in the structure of wood When processing wood, there are often defects in the structure of wood associated with an incorrect structure of the trunk. There are the following types defects in the structure of wood: 1) oblique, or the slope of the fibers, which is the deviation of the fibers from the longitudinal axis of the trunk; 2) roll - solid or local in the form of a sharp thickening of the wood of late annual layers; 3) pilosity - sharply wavy or confused arrangement of wood fibers (wood blanks with such a defect are used in the manufacture of art products, furniture, ax handles and various handicrafts); 4) curl - local curvature of annual layers near knots or sprouts (wood with such a defect is used in furniture production and art crafts); 5) resin pockets. They are found in coniferous wood, especially in spruce, they are cavities between annual layers filled with resin; 6) pitched - a section of coniferous wood, richly impregnated with resin; 7) double core - two cores in one cross section of the log, which are formed at the place of the bifurcation of the trunk; 8) stepson - stunted and dead second peak, which is usually located at an acute angle; 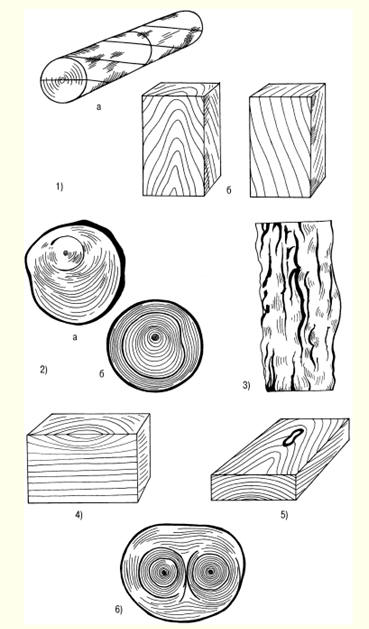
Rice. 3. Defects in the structure of wood: 1 - varieties of fiber inclination: a - tangential inclination in round timber; b - local; 2 - roll: a - solid; b - local; 3 - fibrous pilus in birch; 4 - one-sided curl; 5 - pocket; 6 - double core in a pine trunk; 7 - stepson; 8 - dry side; 9 - pine cancer; 10 - prorost: a - open; b - closed; 11 - false core: a - rounded; b - stellate; c - bladed 9) dryness. Occurs as a result of damage to the bark of a growing tree in the form of a dead section of the trunk; 10) prorost. It is an overgrown wound, usually filled with remnants of the bark and dead tissues; 11) cancer, which is a wound of a tree and occurs on the surface of the trunk as a result of the activity of parasitic fungi and bacteria, while changing the structure of the wood and the shape of the trunk; 12) false core, which resembles a real heartwood, but differs in a more heterogeneous structure and less regular shape, stands out as a dark, unevenly colored zone in the central part of the trunk, is separated from the sapwood by a dark and sometimes light stripe, appears from the impact of fungi, severe frosts, as a reaction to wounds and for other reasons, with the wood of the false core being more brittle and less durable, and the appearance is generally worse; 13) internal sapwood - the presence of several annual layers in heartwood, which are similar in color and properties to sapwood, and it has a reduced resistance to decay and increased permeability to liquids; 14) aquifer - defect of wood in the form of areas with high humidity as a result of the action of bacteria, fungi, the penetration of rainwater through wounds or from oversaturation of the soil with moisture. 4. Fungal lesions When cutting wood, in some cases, mushroom sound spots - abnormally colored areas of the kernel, which are formed in growing trees under the influence of wood-staining or wood-destroying fungi. In felled wood, the further development of this defect stops. Fungal heart spots are observed on the ends in the form of spots of various sizes and shapes of brown, reddish-gray or gray-violet colors. This defect causes: a decrease in impact strength, an increase in water absorption and water permeability, a deterioration in the biostability and appearance of wood; in terms of strength under static load, it almost does not change, and the structure of the affected wood is preserved. When storing wood on raw sapwood, molds often appear - mycelium and fruiting of mold fungi on the surface of the wood in the form of individual spots or a continuous coating, while staining the wood in various colors. Mold does not affect the mechanical properties, but worsens the appearance of wood; after drying, it is easily removed, leaving dirty and colored spots. In felled wood, often formed sapwood mushroom stains - abnormally colored areas of sapwood under the influence of wood-destroying fungi that do not cause rot. Sapwood mushroom stains do not affect the mechanical properties of wood, but worsen its appearance and increase water resistance. By color, blue is distinguished - in the form of a gray color of sapwood with bluish or greenish hues and colored sapwood spots - in the form of orange, yellow, pink and brown color of sapwood. Fungi that stain sapwood can attack adhesives and paintwork. In felled wood during storage in the warm season, as a result of the development of biochemical processes with or without the participation of fungi, such a defect arises as browning. Browning of wood manifests itself in the form of abnormally colored areas of brown hardwood of various shades. Browning is observed on the ends in the form of spots of various sizes and shapes, and on the side surfaces - in the form of elongated spots, stripes or continuous damage to the sapwood, while the appearance of the wood worsens and strength and hardness slightly decrease. To prevent browning of wood, steaming of lumber is carried out. Great damage to wood rot, formed under the influence of fungi. Rots are distinguished by the color and structure of the lesion - variegated sieve, white fibrous; and also by types - sapwood, sound and external rotten. Rotten wood is a source of fungal infection for various wooden structures. Rot develops gradually and has three stages: at the first, only the color of the wood changes; on the second, wood partially changes its structure and hardness under the influence of rot; at the third, the wood completely loses its strength and hardness. Depending on the stage of development of rot and the size of the lesion, the quality of wood can be significantly reduced. 5. Chemical stains, biological damage and warpage In the process of wood processing, such a phenomenon as chemical coloring of wood is often encountered - abnormally colored areas in felled wood resulting from chemical and biochemical processes. In most cases, it is associated with the oxidation of tannins. Typically, such areas are located in the surface layers of wood - at a depth of 1-5 mm. As the practice of wood processing shows, chemical colorings change only its color and gloss, while other properties of wood remain unchanged. With intense natural coloring, the appearance of wood deteriorates, but when it dries, the chemical coloring gradually fades. In case of violation of the storage technology of freshly cut timber, the wood is exposed to biological damage in the form of wormholes - passages and holes made in wood by insects and their larvae (beetles, butterflies, termites, etc.). The optimal conditions for the life of these insects are a temperature of + 18-20 ° C and a relative humidity of 60-80%. Wormholes vary in depth of penetration: superficial (no more than 3 mm deep), shallow (no more than 5 mm in round timber and no more than 5 mm in lumber) and deep. At the same time, they can be non-through and through, i.e., facing two opposite sides of the board. The surface wormhole does not affect the mechanical properties of wood, while the shallow and deep ones violate the integrity of the wood and reduce the mechanical properties. During long-term storage with a violation of technology, a so-called rotten wormhole can form in the wood, which is caused by house pests that can also develop on dry wood - furniture and house grinders, house barbel, termites. In this case, the number of deep passages is large, and the wood inside them turns into a rotten mass with a high content of drilling flour. When drying or moistening, as well as during mechanical processing, as a result of anisotropy of shrinkage - swelling and internal stresses in wood - such a phenomenon is often observed as warp in the form of a change in the shape of the assortment. The warping of sawn timber can be of different types: longitudinal along the face, complex, longitudinal along the edge, transverse, and also like a wing (wingedness) (Fig. 4). The nature of warping depends on sawing it out of a log. Warping reduces the quality of lumber and wood products, complicates processing and cutting, increases the amount of waste, and generally makes it difficult to use wood.  Rice. 4. Types of warping: a - transverse in face; b - longitudinal along the face; c - wingedness The phenomenon of warping is most often observed in lumber obtained by processing birch. 6. Foreign inclusions, mechanical damage and defects in machining In some cases, during the processing of wood, foreign inclusions are found in the form of a foreign body of non-wood origin - nail, wire, metal fragment or stone. An external sign of such a defect can be local swelling and folds of the bark in the wood, a dent, a hole. Such inclusions complicate the mechanical processing of wood and often cause damage to cutting tools - milling cutters, circular saw cutters, etc. Mechanical damage and defects in machining may have a different nature and different origin. Sometimes there is charred wood. The charring of wood is the result of damage to it by fire, while changing its shape, which makes it difficult to use and causes loss of wood. Carra - this is damage to the trunk during tapping, which causes resinification of the wood. Obzol is a part of the side surface of a log that has been preserved on an edged board or part, which leads to a decrease in the actual width of the board and makes it difficult to use. When processing wood with a cutting tool, risks on its surface waviness - non-flat cut or irregularities in the form of arched elevations and depressions as a result of cylindrical milling of wood. Poor-quality wood processing leads to the appearance of surface hairiness in the form of the presence of incompletely separated fibers and moss - the presence of bundles of incompletely separated fibers and small particles of wood. Zarub - local damage to the wood surface with an axe. Gash - local damage to the wood surface by a cutting tool (saw). During the harvesting and processing of timber, there are flakes - lateral cracks extending from the end of the round timber. In similar work, it is often obtained breakouts - recesses with uneven surfaces as a result of local removal of wood when exposed to tools or mechanisms. When processing wood with a cutting tool against the fibers, various mechanical grips are often observed, which leave dents - depressions on the surface formed as a result of local crushing of wood, as well as scratch - damage to the surface in the form of a narrow long recess. As a result, the dents of the cutting edge of the tool are formed scallops - areas of the untreated surface in the form of a narrow strip protruding above the treated surface. When sanding the surface of wood, sometimes a defect such as grinding - removal of part of the wood below the level of the treated surface. With increased friction of cutting tools in the process of wood processing, such a defect often occurs as burn wood in the form of a darkened area of the treated surface. The above wood defects reduce the quality of processing, affect gluing, finishing and veneering of the material or the whole product, in some cases worsen the appearance and violate the integrity of the wood, worsen the mechanical strength and make it difficult to use. LECTURE No. 3. Tree species 1. Key to tree species Based on the "Handbook of wood" A. M. Borovikova и B. N. Ugoleva the determinant of breeds is made. 1. Groups of tree species: 1) annual layers are clearly visible on all cuts of wood. The core rays are not visible. There are no vessels. The wood of some species has resin passages (conifers); 2) annual layers are clearly visible due to the difference in the structure of early and late wood. In the early zone of the annual layers, large vessels form a continuous ring of holes, clearly visible to the naked eye. The late zone of the annual layers is of a dense structure, there are only small vessels. Small vessels and parenchymal cells form a pattern in the form of radial stripes, wavy lines running along the border of annual layers, individual dashes or dots. Most breeds show medullary rays; 3) in most breeds, annual layers are poorly visible. The vessels in the transverse section are not at all visible to the naked eye, or if they are visible, they do not form a continuous ring, but are evenly scattered throughout the annual layer. The late zone of the annual layer has no pattern. In some breeds, core rays are visible - scattered vascular hardwoods; 2. Tree species: 1) conifers: a) resin canals are quite large and numerous. Annual layers are clearly visible in all sections. The core has a color from pink to brownish-red. The sapwood is wide, has a color from yellowish to pale pink (Scotch pine). Further, similarly for the rest of the conifers; 2) annular hardwoods: a) the medullary rays are wide and clearly visible on all cuts. The wood of the core is dark brown or yellowish brown in color. The sapwood is narrow, the color is light yellow. Annual layers are clearly visible on all sections. On a cross section in late wood, light radial flame-like stripes of small vessels are visible. The wood is solid. Further, similarly for other breeds; 3) scattered vascular hardwoods: a) annual layers are poorly visible in all sections. The wood is white with a yellowish or pinkish tint. On the radial section, the core rays are visible in the form of narrow short shiny dark spots. Often there are core repetitions that look like dots or dashes of a reddish-brown color. The wood is quite hard and heavy (birch); b) the wood is white with a slight pink tinge. The annual layers are barely visible. The wood is light, soft (little-leaved linden); c) the height of the medullary rays in the radial section is about 0,5 mm. Annual layers are not clearly visible on all sections, but best of all - on the transverse one. The core rays in the radial section create a characteristic ripple and strong brilliance. The wood is white with a yellowish or pinkish tint, hard, heavy (Nuclear maple); d) there is no nucleus. The wood is white with a slight greenish tint. Sometimes there is a defect - a false core of a brownish color. Annual layers are visible on all sections. There are core repetitions in the form of yellow stripes. The wood is light and soft (aspen). Using the tree species guide, you can determine the type of wood. 2. Main conifers Conifers include spruce, pine, larch, fir, cedar, yew, and juniper, but it grows in the form of shrubs. Ale - non-nuclear breed, its wood is white with a slight yellowish or pink tint. It has resin passages, but low resin. In terms of strength, density and resistance to decay, it is slightly inferior to pine. The annual layers are clearly visible. The most common are two types of spruce - ordinary and Siberian. The first grows in the European part of Russia, the second - from the Urals to Primorye. Spruce is the main raw material for pulp production. The uniformity of the structure and the ability to resonate make it indispensable in the production of musical instruments. From the bark of spruce, tannins are obtained for the leather industry. Pine - sound rock with resin passages. It has a slightly pink heartwood, which becomes brownish-red over time, and a wide yellow-white sapwood. Annual layers are clearly visible on all sections with a sharp transition from early, light to late, dark. Pine has an average density, sufficiently high strength and resistance to decay, and is well processed. Pine wood is used in construction, the production of building parts and furniture, as well as for the manufacture of various parts used in railway transport (in passenger and freight cars), for fastening in mine workings, etc. In addition, pine is also used as a raw material for obtaining cellulose, chipboard and fibreboard, fodder yeast; resin is extracted from it, and biologically active substances are obtained from needles. Larch in Russia it makes up more than half of coniferous forests, which led to its widespread use in construction, furniture production, pulp and paper and hydrolysis industries, etc. Larch has a strong and resilient wood, highly impregnated with resin. Its heartwood is reddish-brown in color, and the sapwood is white or slightly yellowish. The annual rings are clearly visible, with a clear boundary between early and late wood. Larch is slightly knotty, has a high density and strength, is resistant to rotting In Siberia, private houses are built using larch logs (log cabins are made), which last for many years. Fir - the lightest and softest of coniferous tree species. It mainly grows in the northeast of the European part of Russia and from the Urals to the Far East, as well as in the Caucasus. In many ways it looks like spruce, but does not have resin passages. Cedar occupies large areas in Russia, especially in Siberia. It lives up to 800 years and reaches 30 m in height with a trunk diameter of up to 2 m. Cedar wood is light, soft, beautiful in texture and color; has a brownish-pink core and white-pink sapwood; easy to process, resistant to decay; widely used in construction. Pine nuts are the main source of cedar oil, turpentine, medicinal balms. Juniper grows in the form of shrubs, a dense sound rock of brown color with a narrow sapwood. Due to its small size, it is used in small quantities for the manufacture of small turning and carving products. 3. Basic hardwoods Birch is more common in the forests of Russia than other species. Birch - scattered vascular non-core wood with a yellowish tinge. Annual layers are poorly visible. Core rays are visible only on strictly radial cuts (splits). Birch has relatively high strength characteristics, but low resistance to decay; shrinks heavily when dry. Oak - a very valuable vascular rock with a dark brown and yellowish-brown heartwood and a narrow yellowish-white sapwood. On the transverse section in the early zone of the annual layer, large vessels are visible, and in the dark late zone - light radial flame-like medullary rays. Oak wood is dense, durable, resistant to decay, has a beautiful texture; bends well and can be machined. Due to the shortage of this wood, it is used in the form of sliced veneer, as well as in the form of massive parts. In addition to furniture, parquet, barrels for wine and beer, equipment parts in mechanical engineering, etc. are made from oak. Bog oak, which has a dark gray, almost black color, is highly valued in furniture production. From the bark and wood of oak, tanning-extractive substances are obtained, used for dressing leather, fur, etc. Ash - ring-vascular sound rock with yellowish or pink sapwood and light brown heartwood. The annual layers are clearly visible, the core rays are not visible. It resembles oak in color and structure, but is somewhat lighter; used in the country's economy. It is distinguished mainly by high impact strength, it bends well, does not give flakes, therefore it is used in the production of sports equipment: tennis rackets, hockey sticks. Maple - scattered vascular non-nuclear breed. It has white wood with a reddish or brownish tint. Annual layers are clearly visible on all sections, and on the radial - and core rays, which create a characteristic ripple. Maple is used in furniture production and for the manufacture of musical instrument cases, but has limited use due to small reserves in Russian forests. Linden - scattered-vascular breed, non-nuclear. The wood is white with a slight pink tint, the annual layers are hardly visible, it has a homogeneous structure, is soft, cracks little during drying and processing, almost does not warp, therefore it serves as a good material for carving. Nut - a very valuable species, diffusely vascular with wood of brown-gray uneven color, annual layers are weakly visible on cuts, but large vessels are visible. Due to these qualities, walnut wood is used to obtain planed veneer and manufacture highly artistic furniture, various details in order to create original interiors. Poplar - scattered-vascular sound fast-growing breed with a wide sapwood of white color. The annual layers are wide, but inconspicuous. The wood is soft, unstable to decay, used in the production of cellulose and various household products. The reserves of poplar in the forests of Russia are small, so its use is limited. 4. Breeds of limited use For a long time, in the steppe zone of Russia, in rural areas, for the manufacture of simple furniture (chairs, stools, cribs), as well as various crafts (rolling pins, pushers, buttermilk, etc.), such tree species as cherry, pear, apple tree, acacia, hazel, mountain ash, etc. With the development of a market economy in Russia, various arts and crafts have become more active, in which craftsmen in the manufacture of souvenirs, toys, household utensils and children's furniture (cribs, high chairs, etc.) often use the above wood breeds. from wood cherries, which has high strength in combination with the original striping and yellow-brown color, furniture was made with imitation of valuable species (mahogany) and parquet boards. Currently, it is used mainly for the manufacture of various souvenirs and household crafts. Cherry belongs to the sound breed, and this tree grows quickly and can have a height of up to 6 m (Vladimirka-rastunya variety), and the trunk diameter reaches 20-30 cm. Wood pears also has a number of valuable properties - strength, beautiful colors from pinkish-yellow to brownish-red, and the core rays and annual layers are barely noticeable. Pear is a non-nuclear breed, easy to process, has long been used by folk craftsmen for the manufacture of furniture, as well as cases of musical instruments, for household crafts and souvenirs. In rural areas, Russian handicraftsmen have long used hazel (hazel) wood for the manufacture of wooden hoops, boxes, shelves, which is close to birch wood in terms of physical and mechanical properties and also has a white color with a faint sheen. lenses (hazel) refers to a non-nuclear species of the shrub genus. Wood has many valuable properties. mountain ash - high strength, fire resistance, impact resistance. Another advantage is that it consists of a wide sapwood with a beautiful red-white color and prominent annual layers. Craftsmen have long been using this wood to make handles for hammers, ax handles, mallets, simple furniture (stools, chairs, shelves, benches), carved items (balusters, spindles), etc. Rowan is a sound breed. wood apple trees craftsmen of Russia have long been used to make various household utensils, for interior decoration, and also made caskets, souvenirs, cases for musical instruments, etc. This wood has an original color scheme from yellow-pink to reddish-brown, and annual layers and core rays are almost imperceptible. The apple tree belongs to the sound diffuse-vascular breed. 5. Exotic breeds Tree species that grow in countries with a tropical or subtropical climate are classified as exotic species of limited use. Back in the XNUMXth century began to import to Russia, to St. Petersburg blanks of these species for the manufacture of furniture intended for the equipment of the royal palaces, and then the houses of the court nobility. Mahogany wood was most widely used for these purposes. Gradually, in many large cities of Russia, rich people often ordered mahogany furniture for their homes, which was made by first-class cabinetmakers. Of this wood, the most famous is the mahogany species, which grows in Africa. Australia, as well as in Central and South America. The wood of this type of mahogany has a very beautiful combination of colors - from white (narrow sapwood) to red-brown or brownish-red (core). Wood was used in small quantities in Russia ebony. Under this name, blanks from different species, which had black wood, were imported from abroad. Most often, ebony (black) wood was imported, which is heartwood, has a narrow white sapwood and a glossy black heartwood, and in all types of cuts annual layers and core rays are invisible. Ebony wood is used for the manufacture of artistic and decorative products, piano keys, for inlay when decorating interiors, and also woodwind instruments are made from it. The ebony tree (black) grows in India, Africa and Ceylon (in Sri Lanka). Dry density of ebony wood is 1000 kg/m 3, i.e., more than the density of water. Rosewood. In international trade, this name combines different tree species with wood similar in color and structure, growing in the tropics. The wood of such trees is heartwood diffusely vascular, its sapwood is narrow, light yellow, with a grayish tint, the core itself has a purple-brown or chocolate color with a purple tint; it is very heavy, dries out a little, it is difficult to split, but it is well polished. Rosewood wood is used for the manufacture of musical instruments, carvings, turning and other products. Sequoia - the largest tree on the globe, is distinguished by great durability; grows in the tropics, belongs to coniferous species; in terms of physical and mechanical properties, it is close to spruce wood, it is well processed; used in construction, as well as for the manufacture of furniture, pencils. Eucalyptus. In nature, there are more than 500 species, mainly grows in Australia and Oceania. In Russia, eucalyptus grows on the Black Sea coast of the Caucasus in small quantities. Eucalyptus is a fast growing tree, reaching very large sizes - more than 100 m in height. Heartwood diffusely vascular hardwood, the heartwood is brown with various shades, and the sapwood is light. The wood of this tree is dense, has high strength and biostability, is used in construction, car building, etc. LECTURE No. 4. Properties of wood 1. Color, gloss and texture of wood Color wood depends on the climatic conditions of the tree. In a temperate climate, the wood of almost all species is pale in color, and in a tropical climate it has a bright color. The influence of the climatic factor also affects within the same zone, for example, rocks growing in warmer zones - oak, walnut, yew and others, have an intense color, and those growing to the north - spruce, pine, aspen, birch and others, are pale. The color intensity also depends on the age of the trees - with increasing age, the intensity increases. The change in the color of wood occurs under the influence of air and light, as well as from the effects of fungal lesions; when holding wood in water or in special solutions; during steaming and high-temperature drying. The color of wood is an important characteristic and is taken into account when choosing species for the manufacture of furniture, interior decoration, in the production of art crafts, musical instruments, etc. Brilliance - this is the ability of wood to reflect the light flux directionally. Smooth mirror surfaces have the greatest brilliance, as they give a directional reflection. As a rule, the gloss of wood is evaluated by whiteness: the greater the whiteness of the wood, the higher the gloss index. Glare and reflections also give core rays on radial cuts. Texture - this is a natural pattern on tangential and radial cuts of wood, formed by annual layers and anatomical elements. The more complex the structure of wood, the richer its texture. In coniferous wood, the structure is simple and the texture is uniform, it is determined mainly by the width of annual rings and the difference coloration of early and late wood. Hardwood has a complex structure and a richer texture. The nature of the texture largely depends on the direction of the cut. Many species, such as walnut, ash, elm, oak and others, have a beautiful and interesting texture on a tangential cut. The wood on the radial cut also has a beautiful, original texture. The wood of burls formed on the trunks of hardwood trees has high decorative properties. The texture of bird's-eye maple wood, which is created by dormant buds that have not developed into a shoot, is very original. A peculiar and beautiful texture is also created artificially with uneven pressing of wood and its subsequent planing, or when peeling with a wavy knife, or at an angle to the direction of the fibers. With a transparent wood finish, its texture is more pronounced. Texture is the most important indicator that determines the decorative value of wood. Types of wood texture: 1) without a pronounced pattern - linden, pear; 2) finely speckled pattern - oak, beech, plane tree; 3) moire pattern - gray maple, wavy birch, mahogany; 4) drawing "bird's eye" - ash, maple, Karelian birch, Ukrainian poplar; 5) shell pattern - Caucasian walnut, ash, elm - butt part; 6) knotted pattern - spruce, pine. 2. Moisture content of wood and properties associated with its change Freshly cut wood, as a rule, contains a large amount of water, and in the future, depending on the storage conditions, it can increase or decrease, or remain at the same level. But in most cases, it is necessary to take measures to remove water, that is, to dry the wood. An indicator of the water content in wood is humidity, which is divided into absolute and relative. In practice, they mainly use absolute lute value of humidity, which is determined by the formula: Wabs. = [(m - m0)/m0] × 100%, where m is the mass of the wet wood sample, g; m0 - the mass of the same absolutely dry sample, g. The indicator of relative humidity is rarely used, mainly as an indicator of the moisture content of firewood. It is determined by the formula: Wrel. = (m - m0 / m) × 100%. There are two ways to determine humidity - direct and indirect. The direct method is based on the extraction of water from wood. To do this, the cleaned wood sample is dried in an oven at a temperature of 103 ° C until the moisture is completely released. During the drying process, the sample is weighed - the first time after 6-10 hours after the start of drying, and then every 2 hours. Drying is stopped after the weight of the sample no longer decreases. The direct method allows you to determine the moisture content of wood with great accuracy. The second method is indirect, based on measuring the electrical conductivity of wood using an electric moisture meter. With this measurement, the scale of the device shows the amount of humidity. This method makes it possible to quickly determine the humidity. But its disadvantage lies in the measurement error, which is 2-3%, and with a wood moisture content of more than 30% - even higher. Water in wood is in a bound and free state. Bound water is located in the cell walls and is held firmly. The removal of such water is difficult and has a significant effect on changing most of the properties of wood. The maximum amount of bound water corresponds to the cell wall saturation limit, which is taken into account in calculations: Wb.s. = 30%. Free water is located in cell cavities and intercellular spaces, so it is easier to remove from wood. Freshly cut wood has a moisture content in the range of 50-100%, and with a long stay in water - more than 100%. After drying in the open air, the humidity is reduced to 15-20%. Humidity of 20-22% is called transport, and the moisture that the wood has during the period of operation, - operational. Drying wood is of two types - atmospheric, at ambient temperature, and artificial or chamber, when the temperature can be up to 100 ° C and above. During chamber drying, wood shrinkage occurs, i.e., a decrease in linear dimensions in the radial direction by 3-7%, and in the tangential direction - by 8-10%, along the fibers - 0,1-0,3%. The total volumetric shrinkage is 11-17%. When drying wood with a decrease in moisture, its mechanical properties change - elasticity decreases, but compressive strength increases, and electrical conductivity also decreases. 3. Density of wood. Thermal properties of wood Density of wood is the mass per unit volume of the material expressed in g/cm 3 or kg/m 3. There are several indicators of wood density, which depend on humidity. The density of a woody substance is the mass per unit volume of the material that forms the cell walls. It is approximately the same for all breeds and is equal to 1,53 g/cm 3, i.e., 1,5 times higher than the density of water. The density of absolutely dry wood is the mass per unit volume of wood in the absence of water in it. It is determined by the formula: ρ0 = m0 / V0, where p0 - density of absolutely dry wood, g/cm 3 or kg/m 3; m0 - weight of a wood sample at a moisture content of 0%, g or kg; V0 - the volume of the wood sample at a moisture content of 0%, cm 3 or m 3. The density of wood is less than the density of the wood substance, since it has voids filled with air, i.e. porosity, which is expressed as a percentage and characterizes the ratio of voids in absolutely dry wood. The greater the density of wood, the less its porosity. The density of wood significantly depends on humidity. With increasing humidity, the density of wood increases. According to the density, all species are divided into three groups (at a wood moisture content of 12%): 1) rocks with low density - 540 kg/m 3 and less - this is spruce, pine, linden, etc .; 2) rocks of medium density - from 550 to 740 kg/m 3- this is oak, birch, elm, etc .; 3) rocks of high density - 750 kg/m 3 and more - it's dogwood, hornbeam, pistachio, etc. Thermal properties of wood are heat capacity, thermal conductivity, thermal diffusivity and thermal expansion. Heat capacity - the ability of wood to accumulate heat. The specific heat capacity C is taken as an indicator of heat capacity - the amount of heat required to heat 1 kg of wood mass by 1 °C. It is measured in kJ/kg × t °C. Dry wood is a wood substance and air, and the mass fraction of air in it is insignificant. Therefore, the heat capacity of dry wood is almost equal to the heat capacity of wood substance. The specific heat capacity of wood is practically independent of the species and at a temperature of 0 ° C for absolutely dry wood is 1,55 kJ. With an increase in temperature, the specific heat capacity slightly increases and at a temperature of 100 °C it increases by about 25%. When wood is moistened, its heat capacity increases. The process of heat transfer in wood is characterized by two indicators - the coefficient of thermal conductivity and the coefficient of thermal diffusivity. Coefficient of thermal conductivity? numerically equal to the amount of heat that passes per unit time through a wall of wood with an area of 1 m 2 and 1 m thick with a temperature difference on opposite sides of the wall of 1 °C. It is measured in W/(m × °C). The coefficient of thermal diffusivity characterizes the rate of change in the temperature of wood when it is heated or cooled. It determines the thermal inertia of wood, i.e. its ability to equalize the temperature. The thermal diffusivity is calculated by the formula: α = λ/s × ρ, where ρ is the density of the material, kg/m3; λ - coefficient of thermal conductivity, W / (m × °С); c is the specific heat capacity of wood, kJ / (kg × °С). 4. Electrical and acoustic properties of wood As shown by numerous studies of the electrical properties of wood, its electrical conductivity, i.e., the ability to conduct electric current, is inversely related to its electrical resistance. There are surface and volume resistances, which together give the total resistance of a wood sample placed between two electrodes. Volume resistance characterizes the obstacle to the passage of current through the thickness of the sample, and surface resistance - along the surface. Indicators of electrical resistance are specific volume and specific surface resistance. Studies have shown that dry wood conducts electricity poorly, but with increasing humidity, its resistance decreases. This can be seen from the data obtained during the studies (Table 1). Table 1 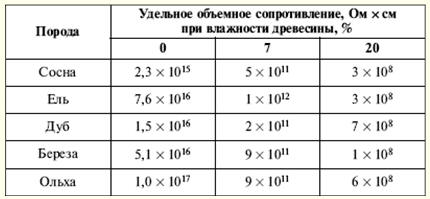 A decrease in surface resistance occurs with an increase in humidity. For example, with an increase in beech moisture content from 4,5 to 17%, the surface electrical resistance decreases from 1,2 × 1013 up to 1 × 107 Ohm. In addition, as a result of research, it was found that a decrease in the electrical resistance of wood occurs when it is heated, especially at its low humidity. Thus, an increase in temperature from 20 to 94 ° C reduces the resistance of absolutely dry wood by 10 6 time. acoustic properties. When studying the acoustic properties of wood, it was found that the speed of sound propagation in wood is the greater, the lower its density and the higher the modulus of elasticity. The average values of the speed of sound along the fibers for room-dry wood are: oak - 4720 m/s, ash - 4730 m/s, pine - 5360 m/s, larch - 4930 m/s. Further studies have shown that the speed of sound across the fibers is 3-4 times less than along the fibers. The speed of sound propagation depends on the properties of materials and, first of all, on density, for example, in steel, sound propagates at a speed of 5050 m/s, in air - 330 m/s, and in rubber - 30 m/s. Based on the data obtained in the study of the acoustic properties of wood, an ultrasonic method for determining its strength and internal hidden defects was built. According to research data, the sound absorption capacity of wood is low, for example, the sound insulation of pine wood with a thickness of 40 cm is 48 dB, and oak with a thickness of 3 cm is 12 dB. As established by research, the best acoustic properties in terms of the greatest sound emission are spruce, fir and cedar wood, which is used for the manufacture of many musical instruments: plucked, bowed, keyboards, etc. As practice has shown, long-term exposure wood has the best acoustic properties - for 4,5 years or more. 5. Durability of wood The mechanical properties include the strength and deformability of wood, as well as some technological properties. The strength of wood is its ability to resist destruction under the influence of external loads. The tensile strength of wood is determined by testing samples for compression, tension, bending, shear. When testing wood for compression, the load is carried out along the fibers, then across and in one place. The tensile strength is determined in MPa by the formula: бsqueeze = PMax /a×b, where PMax - maximum breaking load, N; a and b are the dimensions of the wood sample, mm. According to the test data, it was found that when wood is stretched across the fibers, the strength is approximately 1/20 of the tensile strength along the fibers. Therefore, when designing products and constructing various building structures, cases are not allowed for tensile loads to be directed across the fibers. In practice, in most cases, wood products work with bending loads. Therefore, wood samples must be tested for bending, while determining the tensile strength in MPa according to the formula: бof = 3PMax × l/2 × b × h2, where l - distance between supports, mm; b - sample width in the radial direction, mm; h is the height of the sample in the tangential direction, mm. When the sample is bent on the convex side, tensile stresses arise, and on the concave side, compression stresses arise. At loads above the limiting value, the destruction of wood occurs in the form of a rupture of stretched fibers on the convex side of the fracture of the sample. Shear strength is of great importance. This indicator is determined when testing three types of shear: for shearing along and across the fibers; for cutting wood across the grain. At the same time, the tensile strength of wood for chipping is bsk, MPa is determined by the formula: бsk = PMax /b×l, where P Max - maximum load, N; b, l - thickness and length of the sample in the shearing plane, mm. Tests for cutting wood across the fibers are carried out on samples using a movable knife. In this case, the tensile strength in MPa is determined by the formula: τ = PMax / 2 × a × b, where PMax - maximum load, N; a and b are the dimensions of the sample section, mm (transverse). As the test results show, the strength of wood when cut across the fibers is 4 times greater than when chipped along the fibers. As tests have shown, the moduli of elasticity in compression and tension of wood are approximately the same and amount to 12,3 GPa for pine, 14,6 GPa for oak and 16,4 GPa for birch at a moisture content of 12%. The modulus of elasticity across the fibers is about 20-25 times less than along, and in the radial direction is higher than in the tangential direction, by about 20-50%. When testing wood, the modulus of elasticity is also determined: E = 3 × P × l / (64b × h3 × f), where P is the load equal to the difference between the upper and lower measurement limits, N; l - distance between the supports (on which the wood sample is located), mm; b and h - sample width and height, mm; f - deflection equal to the difference between the arithmetic mean values of the deflection at the upper and lower loading limits, mm. 6. Technological properties of wood Technological properties: impact strength, hardness, wear resistance, ability to hold screws, nails and other fasteners, as well as machinability with cutting tools. Impact strength of wood - this is its ability to absorb forces (work) upon impact without destruction. The greater the amount of work required to break the sample, the higher its viscosity. Impact strength is determined by the formula: A \uXNUMXd Q / b x h, J / cm 2, where Q is the work expended on the fracture of the sample, J; b and h are the width and height of the sample. Hardness of wood is its ability to resist indentation of a body made of a harder material - a steel punch with a hemispherical tip of radius r = 5,64 mm to a depth of 5,64 mm. At the same time, at the end of loading, the load R is counted on the scale of the machine's force meter. After the test, an imprint of 100 mm area remains in the wood 2. The static hardness of the sample is determined in N/mm using the formula: H \uXNUMXd P / π × r2, where π×r2 - the area of the imprint in the wood when a hemisphere of radius r is pressed into it, mm. If there is a splitting of the samples during the testing process, then the punch is pressed to a smaller depth - 2,82 mm, and the hardness is determined by the formula: H = 4P / (3π × r2). All rocks are divided into three groups according to the hardness of the end surface: soft - with a hardness of 40 N / mm 2 and less, hard - 41-80 N/mm 2 and very hard - more than 80 N/mm 2. Durability wood characterizes its ability to resist wear when rubbing against the surface of abrasive elements or microroughness of a more solid body. When testing for abrasion, conditions are created that mimic the actual process of abrasion of wood used for floors, stairs, decking. Abrasion is carried out on a special machine. In this case, the abrasion index t is calculated in mm according to the formula: t = h × (m1 - M2)/m1, where h is the sample height before abrasion, mm; m 1 and m 2 - the mass of the sample, respectively, before and after the test, g. The specific resistance to pulling out a nail or screw is determined by the formula: Рud. = PMax / l (N/mm), where PMax - maximum load when pulling out nails or screws; l is the length of driving a nail or screwing a screw. The ability of wood to hold fasteners depends on its species, density and moisture content. The pull-out resistance of nails hammered in the radial and tangential directions is approximately the same, but it is higher than when nails are driven into the end of the sample. The ability of wood to bend - the best in beech, oak, ash, worse - in conifers. To improve the pliability of wood, it is steamed before bending, then after bending it is cooled and dried in a fixed state, as a result of which it acquires a stable curved shape. The ability of wood to split - this is the process of separating it along the fibers under the action of the load transmitted to the wedge. This is a negative property of wood when driving nails close to the edge, as well as crutches, screws when screwing in, but a positive one when chopping firewood or harvesting split logs. LECTURE No. 5. Alloys 1. The structure of metals Metals and their alloys - the main material in mechanical engineering. They have many valuable properties, mainly due to their internal structure. Soft and ductile metal or alloy can be made hard, brittle, and vice versa. In order to consciously change the properties of metals, it is necessary to know the basics of their crystal structure. As is known, all bodies consist of a large number of atoms, which are held together by cohesive forces, oscillating at a high frequency near the points of equilibrium. Since the atoms of different metals are different, each metal has its own specific properties. These properties depend on the arrangement of atoms among themselves, the nature of their bonds, and the distance between them. If you change the distance between the atoms or the order of their arrangement, the properties of the metal will also change. In amorphous bodies - resin, glass, rosin, etc. - the atoms are arranged randomly. In metals, they are in a certain geometric order, forming crystals, therefore metals are crystalline bodies. Metals differ not only in the order of arrangement of atoms, but also in the crystal lattice, which is an imaginary spatial grid consisting of elementary cells, at the nodes of which there are atoms. The following crystal lattices of metals with dense packing of atoms are distinguished: cubic body-centered, cubic face-centered and hexagonal. In a cell of a cubic body-centered lattice, atoms are located at the vertices and the center of the cube. Such a cell contains nine atoms (chromium, tungsten, vanadium, molybdenum, lithium, and at certain temperatures, iron and other metals). In a cell of a cubic face-centered lattice, atoms are located at the vertices of the cube and at the intersection of the diagonals of each plane. Such a cell has 14 atoms (lead, nickel, copper, gold, silver, plate, iron at certain temperatures, and other metals). In a cell of a hexagonal crystal lattice, atoms are located at the vertices and in the center of the hexagonal bases of the prism, and three atoms are located in its middle plane, while such a cell contains 17 atoms (magnesium, zinc, cadmium, osmium, beryllium and other metals). Under certain conditions, some metals - iron, titanium, zirconium, strontium, cobalt, calcium and others - can be rearranged from one type of crystal lattice to another, for example, from cubic body-centered to face-centered and even hexagonal. The elementary cell displays only one element, or one cell, of the crystal lattice. The entire crystal lattice in a real metal consists of a large number of repeatedly repeating elementary cells. Of great importance is the distance between the atoms of a cell of a crystal lattice or between parallel atomic planes that form an elementary cell. The greater this distance, the less durable the metal. The distance between them is measured in angstroms - 1 A = = 10 -8 cm or in nanometers - 1 A \u0,1d XNUMX nm. From practice it is known that iron is stronger than copper, and copper is stronger than aluminum. 2. Crystallization and structure of metals and alloys The arrangement of atoms - the type of crystal lattice - the natural property of the metal, the shape of the crystals and their sizes depend on the process of the transition of the metal from a liquid to a solid state. The process of crystal formation during the solidification of metals is called crystallization. During the crystallization of metals, heat is released, and during the transition of metals from a solid to a liquid state, heat is absorbed. Observations with the help of temperature-measuring partings of the process of temperature decrease during the transition of a metal from a liquid state to a solid state, they made it possible to establish a certain regularity. First, the temperature drops evenly. In the initial period of crystal formation, due to the release of latent heat during the formation of the crystal lattice, the temperature drop stops, and it remains unchanged until the metal is completely solidified. After all the metal has hardened, the temperature starts to drop again. The temperature corresponding to a horizontal area is called critical. The crystallization of metals is similar to the crystallization of salts, and this process consists of two elementary processes occurring simultaneously. The first is the formation of crystallization centers, or crystal nuclei, the second is the growth of crystals from these centers. The first stage - the appearance of nuclei of metal crystals. The second stage - as the metal cools, more and more liquid metal atoms join the nuclei, which are grouped in a certain order one near the other, forming elementary cells of the crystal lattice. This process continues until the end of crystallization. Moreover, the crystals of the solidified metal have an irregular and very diverse shape, which is explained by the conditions of crystallization. In the process of crystallization, the number of crystals increases - in 1 mm 3 over 1000 crystals can be formed. Crystals that have an irregular external shape are called crystallites, or grains. Pure metals are relatively rarely used in mechanical engineering and other branches of the economic complex. More widely used are alloys consisting of two or more elements (two metals, such as copper and zinc, or a metal and a non-metal, such as iron and carbon). The elements in an alloy are called components. Depending on the arrangement of atoms in the crystal lattice, substitutional solid solutions and interstitial solid solutions are distinguished. In a substitutional solid solution, the atoms of the soluble component are replaced by solvent atoms, while in the interstitial solid solution, the solvent atoms are located between the atoms of the soluble component at the weakest points of the crystal lattice elements. Alloys, which are solid solutions, have valuable properties. They are harder and stronger than the components included in it. The components of some alloys during crystallization can enter into a chemical bond, forming a chemical compound. Chemical compounds have very high hardness and good electrical resistance. 3. Diffusion and diffusionless transformations Under diffusion understand the movement of atoms in a crystalline body at distances exceeding the average interatomic distances of a given metal. If the movement of atoms is not associated with a change in concentration in individual volumes, then such a process is called self-diffusion. Diffusion accompanied by a change in concentration is called heterodiffusion. In cases where heterodiffusion is accompanied by the formation of new phases, which most often occurs during chemical and technical processing, it is called reactive diffusion. The diffusion process is based on an atomic mechanism in which each atom performs more or less random walks. Diffusion transformations in metals occur during various chemical and thermal treatments - chrome plating, carburizing, aluting (aluminizing), etc. Chrome provides increased heat resistance of steel up to 800 °C, high corrosion resistance in environments such as fresh and sea water, acetic and phosphoric acids, and erosion resistance at low and high temperatures. Chromium plating of steels containing more than 0,3-0,4% carbon also increases hardness and wear resistance. During chromium plating, the diffusion layer consists of a solution of chromium in? - iron, and the chromium content on the surface is 25-50%. In this process, in the case of using CrCl 2 the following reaction takes place: CrCl 2 + Fe → FeCl 2 +Cr. During heat treatment of steel, non-diffuse, or allotropic, transformations in the process of secondary crystallization. In particular, at a temperature of +775 ° C in steel containing 0,6% carbon, allotropic transformations begin, i.e., the separation of ferrite from austenite (a solid solution of carbon (up to 2,14%)) and other impurities in the volume of iron. Ferrite - a solid solution of a small amount of carbon (up to 0,04%) and other impurities in? - gland - a soft, plastic and insufficiently strong structural component. Since ferrite contains a negligible amount of carbon, the remaining austenite will gradually, as the ferrite precipitates, be enriched in carbon. When the carbon concentration in the remaining austenite reaches 0,8%, at a temperature of +727 ° C, steel containing 0,6% carbon will contain ferrite and austenite, and at temperatures below +727 ° C - ferrite and pearlite, and the ferrite-pearlite structure will remain without significant changes even with further cooling of the steel down to room temperature. Similar transformations are characteristic of all hypoeutectoid steels (containing less than 0,8% carbon). The difference will be only in the temperatures of the beginning of ferrite precipitation. Moreover, if the steel contains 0,8% carbon, its secondary crystallization will proceed at a constant temperature (+727 °C) and be accompanied by only one process - the formation of pearlite. This is explained by the fact that in this case the carbon content in the steel corresponds to the eutectoid composition - a mechanical mixture of crystals released from the liquid alloy at the same time. This creates a fine-grained structure of the alloy. 4. Classification of alloys. Iron and its alloys Steel and cast iron - basic materials in mechanical engineering. They make up 95% of all alloys used in technology. Steel is an alloy of iron with carbon and other elements containing up to 2,14% carbon. Carbon - the most important impurity of steel. The strength, hardness and ductility of steel depend on its content. In addition to iron and carbon, steel contains silicon, manganese, sulfur and phosphorus. These impurities enter the steel during the smelting process and are its inevitable companions. Cast iron - iron-based alloy. The difference between cast iron and steel lies in the higher carbon content in it - more than 2,14%. The most widespread are cast irons containing 3-3,5% carbon. The composition of cast irons includes the same impurities as in steel, i.e. silicon, manganese, sulfur and phosphorus. Cast irons, in which all carbon is in chemical combination with iron, are called white (according to the type of fracture), and cast irons, all or most of which carbon is graphite, are called gray. In white cast irons, there is always one more structural component - ledeburite. This is a eutectic, i.e. a uniform mechanical mixture of austenite and cementite grains, obtained during crystallization, it contains 4,3% carbon. Ledeburite is formed at a temperature of +1147 °C. Ferrite - a solid solution of a small amount of carbon (up to 0,04%) and other impurities in? - iron. It is practically pure iron. Cementite - chemical compound of iron with carbon - iron carbide. Perlite - uniform mechanical mixture in an alloy of ferrite and cementite. This mixture received such a name because the section during its etching has a mother-of-pearl hue. Since pearlite is formed as a result of secondary crystallization processes, it is called a eutectoid. It is formed at a temperature of +727 °C. It contains 0,8% carbon. Perlite has two varieties. If the cementite in it is in the form of plates, it is called lamellar, but if the cementite is in the form of grains, the perlite is called granular. Under the microscope, cementite plates appear shiny because they are very hard, polish well, and corrode less when etched with acids than soft ferrite plates. If iron-carbon alloys are heated to certain temperatures, an allotropic transformation of α-iron into ν-iron will occur and a structural component is formed, which is called austenite. austenite is a solid solution of carbon (up to 2,14%) and other impurities in ν-iron. Ability of carbon dissolve in iron is not the same at different temperatures. At a temperature of +727 °C, ν-iron can dissolve no more than 0,8% of carbon. At the same temperature, austenite decomposes to form pearlite. Austenite is a soft structural component. It is characterized by high plasticity, does not have magnetic properties. When studying the structural components of iron-carbon alloys, it was found that at room temperature they always consist of two structural elements: soft ductile ferrite and hard cementite, which strengthens the alloy. 5. State diagrams of alloys Alloys can be obtained by combining most metals with each other, as well as with non-metals. State diagrams of alloys give a visual representation of the transformations occurring in alloys depending on their chemical composition and temperature. When constructing diagrams of the state of alloys, the chemical composition or concentration of the alloy as a percentage is indicated on the abscissa axis. To do this, a horizontal line of a certain length is divided into one hundred identical parts, and each division is taken as 1% of one of the alloy components.  Rice. 5. Diagram of the state of alloys of the lead-antimony (Pb-Sb) system Point A corresponds to pure lead, and point B corresponds to pure antimony. The temperature is indicated on the y-axis on a certain scale. In order to build an alloy state diagram, first a series of cooling curves for alloys of the same elements with different concentrations is built. Based on these curves, a diagram is built. Alloys, the components of which, during solidification, form only mechanical mixtures, belong to the first group. The diagram of these alloys is conditionally called the phase diagram of the first kind. The diagram of alloys that form only solid solutions during solidification is called the phase diagram of the second kind. The most typical for diagrams of the first kind are alloys of lead with antimony. Construction of a diagram (of the first kind) of the state of Pb-Sb alloys: 1) cooling curves of hypoeutectic alloys; 2) state diagram of Pb-Sb alloys; 3) cooling curves of hypereutectic alloys. The diagram is built for five types of lead-antimony alloy: 1) 5% antimony and 95% lead; 2) 10% antimony and 90% lead; 3) 20% antimony and 80% lead; 4) 40% antimony and 60% lead; 5) 80% antimony and 20% lead. They all have two critical temperatures: top and bottom. The study of the crystallization processes of these alloys shows that the upper critical temperature corresponds to the beginning, and the lower - to the end of alloy solidification. Thus, the process of crystallization of Pb-Sb alloys differs sharply from the crystallization of pure metals. Alloys crystallize in the temperature range, and pure metals - at a constant temperature. The mechanical mixture of crystals released from a liquid alloy at the same time is called eutectic (translated from Greek - "well built"). Alloys of this concentration are called eutectic. The DIA line on the diagram is called liquidus line (translated from Greek - "liquid"). Above this line, any lead-antimony alloy is in a liquid state. The DSVE line was named the line solidus (translated from Greek - "solid"), or eutectic line. Point C shows the composition of the eutectic. Alloys located to the left of this point are called hypoeutectic, to the right of her hypereutectic. In the structure of hypoeutectic alloys, in addition to eutectic, there is always a certain amount of lead, and in hypereutectic alloys, in addition to eutectic, antimony. LECTURE No. 6. Mechanical properties of metals 1. Deformation and destruction Load application calls deformation. At the initial moment, loading, if it is not accompanied by phase (structural) changes, causes only elastic (reversible) deformation. Upon reaching a certain stress, the deformation (partially) becomes irreversible (plastic deformation), while the structure of the metal and, consequently, its properties also change irreversibly. The dependence of deformation on stress is depicted by the so-called tension diagram. Conditional stress: σ = P / F0 (kgf/mm2), where P is the force; F0 - initial section, and the abscissa axis - relative deformation: ε = ∆l / l, where Δl is the length increment, l - initial length. The tangent of the angle of inclination is straight: tg α \uXNUMXd σ / ε \uXNUMXd E - the modulus of normal elasticity (in kgf / mm 2) - characterizes the rigidity of the material (resistance to elastic deformation), which is determined by the forces of interatomic interaction, depending in the first approximation on the melting temperature of the metal. Since alloying and heat treatment have very little effect on the melting point, the modulus of normal elasticity can be considered as a structurally insensitive characteristic. For all steels E ≈ 2 × 10 4 kgf/mm 2, and for aluminum alloys E ~ 0,7 × 10 4 kgf/mm 2. The conditional stress at which the proportional relationship between ε and σ is violated is the elastic limit (or proportionality limit). For technical purposes (except for elastic elements), a small deviation from proportionality is not considered significant, and it is usually considered that plastic deformation occurs when the permanent irreversible deformation εpl. becomes 0,2%. The conditional voltage at which = 0,2% is called yield strength (on the diagram - σ0,2) and characterizes the resistance of the material to small plastic deformation. The true stress reaches its maximum value at point Z - at the final destruction of the sample. For high-strength and low-plasticity materials σВ > 150 kgf/mm 2, the relative narrowing ψ (change in narrowing) at the point of rupture (destruction) is less than 40%, and ψ is determined by the formula: ψ = (Fо - Fх)Fо, where F 0 - section of the sample before destruction; Fx - section at the moment of maximum deformation. Destruction can be of two types, which can be called "separation" (repture) and "destruction" (vacation). Separation is typical for highly plastic materials (usually high-purity metals), the deformation of which after reaching the point ? В leads to 100% narrowing without the formation of a fracture surface. In all other cases, the narrowing reaches a certain value, after which the sample is destroyed with the formation of fracture surfaces.  Rice. 6. Types of torn samples: a - separation; b - destruction with preliminary plastic deformation; c - failure without preliminary plastic deformation. The destruction process is preceded by: elastic deformation and plastic deformation. 2. Mechanical properties of metals Mechanical properties metals are determined by the following characteristics: elastic limit σТ, yield strength σЕ, tensile strength relative elongation σ, relative narrowing ψ and modulus of elasticity E, impact strength, endurance limit, wear resistance. Hardness, determined by the simplest non-destructive methods, depends mainly on the carbon content and the conditions of heat treatment of steel. For a rough estimate of strength, the following relationship can be used: σВ = HB/3. All metal parts of machines during operation are exposed to various external loads, which can be carried out smoothly, gradually (statically) or instantly (dynamically). Acting on parts, external loads change their shape, i.e. deform The property of materials made of metal and alloys to take their original shape after the termination of the action of external forces is called elasticity, and the deformation that disappears after the load is removed is called elastic. If great efforts are applied to a metal part and after the termination of their action it does not take its original shape, but remains deformed, then such a deformation is called plastic. The ability of metallic materials and parts to deform under the influence of external loads without collapsing, and to retain the changed shape after the termination of the force is called plasticity. Materials made of metals that are not capable of plastic deformation are called fragile. An important property of materials and parts made of metals, along with elasticity and plasticity, is strength. Metal parts or tools, depending on the working conditions, must have certain mechanical properties - strength, elasticity, plasticity. During long-term operation, metal parts of machines are subjected to re-variable loads (stretching - compression). At stresses below the yield strength or elastic limit, they can suddenly fail. This phenomenon is called metal fatigue. The endurance limit (fatigue) is the maximum stress that materials and metal parts can withstand without breaking, with a sufficiently large number of repeated variable loads (cycles). For steel samples, this characteristic is set at 10 million cycles, for non-ferrous metals - at 100 million cycles. Endurance limit is denoted by a Greek letter? -1 and measured in Pa. During operation, many machine parts are heated to high temperatures, reaching 1000 °C or more. For such parts, an important characteristic is heat resistance - the ability of materials made of metals and alloys to maintain the required strength at high temperatures. In metals and alloys that work for a long time under load at high temperatures, there is a phenomenon creep, i.e., continuous plastic deformation under the action of a constant load (metal "creeps"). 3. Methods for strengthening metals and alloys Surface hardening of metals and alloys is widely used in many industries, in particular in modern engineering. It allows to obtain high hardness and wear resistance of the surface layer while maintaining a sufficiently ductile core, improves durability and fatigue strength. Some surface hardening methods are highly productive. In some cases, they are used with great efficiency instead of conventional heat treatment methods. There are a large number of parts, the properties of the surface layer of the metal of which are subject to other requirements than the properties of the inner layers. For example, gear teeth experience strong friction during operation, so they must have high hardness, but have low hardness and good toughness so that the teeth do not collapse from shocks and impacts. Therefore, gear teeth must be hard on the surface and viscous in the core. The most common way to harden the surface layer of metals and alloys is surface hardening, at which only a part of the surface layer of the parts acquires high hardness. The rest is not hardened and retains the structure and properties that were before hardening. At present, surface hardening with induction heating by high-frequency currents is most widely used. This high-performance progressive method of heat treatment provides an increase in the mechanical properties of steel, including yield strength, fatigue and hardness, eliminates the possibility of decarburization, reduces the risk of oxidation of the surface of products and their deformation. Details of complex shape, band saws, cutting tools (milling cutters, drills), levers, axles are subjected to pulse surface hardening. To do this, the hardened part of the part is heated to a temperature exceeding the temperature of conventional heating of this material for hardening, and then cooled at a high rate due to heat removal to the rest of the part mass without the use of cooling media. As a result of impulse hardening, a hardened "white" layer is obtained, which is stable when tempered up to a temperature of 450 ° C, has a fine-grained structure, high hardness and wear resistance. LECTURE No. 7. Iron-carbon alloys 1. Diagram of iron-cementite The iron-cementite diagram covers the state of iron-carbon alloys, which contain up to 6,67% carbon.  Rice. 7. Diagram of the state of iron-carbon alloys (solid lines - Fe-Fe system 3 C; dashed - Fe-C system) carbon steels - these are iron alloys containing up to 2,14% carbon. Steels containing up to 0,8% carbon are called hypoeutectoid, 0,8% carbon - eu-tectoid, over 0,8 to 2,14% - hypereutectoid. White cast irons - these are iron alloys containing from 2,14 to 6,67% carbon. With a content of 2,14 to 4,3% carbon, white cast irons are called hypoeutectic, with 4,3-6,67% - hypereutectic. The iron-cementite diagram shows the state of this alloy during primary and secondary crystallization. These processes are characterized by ASV and AECF curves. DIA curve - line liquidus - displays the temperatures at which the solidification of iron-carbon alloys begins. AECF curve - line solidus - corresponds to the temperatures at which the crystallization process ends. The AE line refers to steels and the ACF line to white cast irons. Point A characterizes the melting point of pure iron - +1539 °C, and point B - the melting point of cementite - +1600 °C. Point E corresponds to the maximum amount of carbon that can be dissolved in austenite at high temperatures. Point C indicates the composition of the eutectic, it corresponds to the content of 4,3% carbon in the alloy. The eutectic formation temperature is +1147 °C. The ECF line is called eutectic, since at any point a eutectic (ledeburite) is formed. On the CF line (hypereutectic cast irons), the component that is excessive in relation to the eutectic, i.e., cementite, will be released from the liquid alloy. Since cementite is formed during primary crystallization, it is called primary. A eutectic arises on the CF line - ledeburite. Consequently, as a result of primary crystallization, hypereutectic cast irons will consist of primary cementite and ledeburite. The ECF line (+1147 °C) is called eutectic, since the formation of a mechanical mixture of austenite and cementite - ledeburite occurs on it. Ledeburite has a eutectic composition, therefore, its crystallization proceeds at a constant temperature of +1147 °C. As a result of primary crystallization, the steel acquires an austenite structure characterized by good ductility and toughness. Therefore, such steel lends itself well to pressure treatment at high temperatures. White cast irons contain brittle and hard ledeburite, which excludes the possibility of pressure treatment even at high temperatures. The PSK line on the diagram characterizes the temperature. at which the processes of secondary crystallization are completed. For the steels shown in the diagram, this temperature is +727 °C. At temperatures below +727 °C, no significant transformations are observed in steels; the structure obtained at +727 °C is retained upon further cooling of the alloy (down to room temperature). The PSK line is called eutectoid. Point S of the diagram corresponds to the composition of the eutectoid - perlite. 2. Steels: classification, free-cutting steels Steels serve as the material basis for mechanical engineering, construction and other industries. Steels are the main raw material for the production of sheet and profile products. By production method steels are divided into Bessemer, converter (with oxygen purge), open-hearth, electric steel, crucible and steel obtained by direct reduction from enriched ore (pellets); by chemical composition - for carbon and alloyed; by appointment - on structural, tool, automatic and steel with special properties. Steels always contain various impurities. The less harmful impurities, the higher the quality of steel. Depending on the quality, steels are distinguished ordinary quality, high quality, high quality and extra high quality. Carbon steels of ordinary quality are among the cheapest and widely used. Depending on the purpose, carbon steels of ordinary quality are divided into three groups: A - supplied by mechanical properties, B - supplied by chemical composition, and C - supplied by mechanical properties and chemical composition. Depending on the normalized indicators (strength characteristic, chemical composition), steel of each group is divided into categories: group A - 1, 2 and 3; group B - 1, 2nd; group B - 1, 2, 3, 4, 5, 6th. Group A includes steel of the following grades: St 0, St 1 kp, St 1 ps, etc. up to ST aux. The letters "St" mean "steel", the numbers from 0 to 6 - the conditional number of the brand, characterizing the mechanical properties of steel. With an increase in the grade number, the ultimate strength σ increasesВ and yield strength σТ and the relative elongation decreases. To indicate the degree of deoxidation, indices are put after the brand number: kp - boiling, ps - semi-calm, cn - calm (for example, St 3 kp, St 3 ps, St 3 cn). Group B includes steels of the following grades: Bst 0, Bst 1 kp, etc. up to Bst 6 kp. Group B steel has two categories. The first category includes steel of all grades containing the following chemical elements: carbon, manganese, silicon, phosphorus, sulfur, arsenic, nitrogen. The second category includes steel grades from BST 1 to BST 6, which include chromium, nickel and copper. Group B includes steel grades VST 1, VST 2, VST 3, VST 4 and VST 5. The indices ps, sp and kp added to the grade indicate the degree of steel deoxidation, for example: VST 3 sp, VST 3 gps, etc. Letter "g" after the number indicates an increased content of manganese. For automated metal-cutting machine tools, the metallurgical industry produces special free-cutting steels capable of forming brittle, easily descending and easily removed chips. It is a steel of increased and high machinability. The high machinability of such steels is achieved by increasing the content of sulfur and phosphorus (up to 0,35%), as well as the introduction of lead (up to 0,35%). Automatic steels are used in large-scale and mass production. Non-critical parts for cars and tractors (fasteners, axles, bushings, etc.) are made from them. 3. Cast irons: white, gray, high strength, malleable Cast iron - the primary product of the processing of iron ores by smelting in blast furnaces. In the structure of cast irons, there can be different components, depending on what part of the carbon is in a structurally free state. This also determines the name of cast iron: white, gray, high-strength, malleable. Cast iron is the most common iron-carbon casting material containing over 2% carbon, up to 4,5% silicon, up to 1,5% manganese, up to 1,8% phosphorus and up to 0,08% sulfur. Cast iron has high casting properties, therefore it is widely used in foundry production as a structural material. Plain bearings are made from cast iron, which has a low coefficient of friction. White cast iron is an alloy of iron with carbon in the form of iron carbide Fe 3 C, i.e., carbon is in a bound state in the form of a chemical compound - cementite. The carbon content in white cast iron ranges from 2,14 to 6,67%, and the primary structure of white cast irons may contain ledeburite, austenite and primary cementite. In addition, the microstructure of white hypoeutectic cast irons includes perlite, secondary cementite and ledeburite at room temperatures. With a content of 2,14 to 4,3% carbon, white cast irons are called hypoeutectic, at 4,3% - eutectic and at 4,3-6,67% - hypereutectic. Gray cast iron widely used in mechanical engineering. It got its name from the gray color of the fracture, due to the presence of free carbon in the form of graphite in the cast iron structure. The metallurgical industry produces eleven grades of gray cast iron: SCh 10 - parts for which the strength characteristic is not mandatory are made from it - shutoff valves (valves, valves, gate valves), pans, lids, and so on; SCH 15, SCH 18 - levers, pulleys, flanges, sprockets, body lightly loaded parts are made from them. High-strength cast iron obtained by introducing magnesium - up to 0,9% and cerium - up to 0,05% into liquid gray cast iron before pouring it into molds. Ductile iron has a higher carbon and silicon content and a lower manganese content. This cast iron combines the valuable properties of steel and cast iron. The designation of their grades includes two numbers - the first indicates the tensile strength, the second - relative elongation. In total, ten grades of high-strength cast iron are produced. For example: HF 38-17, HF 42-12, HF 45-5, HF 50-7, HF 100-2, HF 120-2. Ductile irons are used to make many parts, including shaped parts, machine bodies and beds, sleeves, cylinders, gears, etc. Release of 11 grades of ductile iron, and it is marked according to the same principle as high-strength. Ductile cast irons can have a ferritic, pearlitic, and ferrityl-pearlitic metal base. Ferritic cast iron KCh 35-10 and KCh 37-12 are used for the production of parts operated under high dynamic and static loads - crankcases, gearboxes, hubs, etc., and cast iron grades KCh 30-6 and KCh 33-8 - for the manufacture of less critical parts - clamps, nuts, valves, blocks, etc. LECTURE No. 8. Methods of processing metals 1. Influence of alloying components on transformations, structure, properties of steels Alloying components or elements introduced into steel, depending on their interaction with carbon in iron-carbon alloys, are divided into carbide-forming and non-carbide-forming. The former include all elements located in the periodic system of elements to the left of iron - manganese, chromium, molybdenum, etc. To the right of iron are elements that do not form carbides - cobalt, nickel, etc. The first, as well as the second, alloying elements dissolve in α- or ν-iron, however, the content of carbide-forming elements in these phases of iron is less than that introduced into steel, since a certain amount of them binds to carbon. In this case, the dissolution of alloying elements in the α- and ν-phases leads to a change in the period of the crystal lattice. Elements with a large atomic radius increase it (W, Mo, etc.), and with a smaller one (Si) - reduce it. When the atomic sizes are close (Mn, Ni, Cr), the periods of the crystal lattice change slightly. Studies show that the strength of ferrite varies in proportion to its lattice period. Carbides in alloy steels are solid solutions based on one or another compound: Fe 3 C, Fe 3 Mo 3 C, Fe 3 W 3 C, etc. In alloy steels, two groups of carbides are distinguished: group I - M 3 C, M 23 C 6M 7 C 3 and M 6 C and group II - MC, M 2 C (M - alloying component - element). Group I carbides have a complex crystal lattice and, with appropriate heating, are fairly well soluble in austenite. Group II carbides have a simple crystal lattice, but only partially dissolve in austenite and at a very high temperature. Non-carbide-forming elements (alloyed) are contained in alloyed steels in the form of a solid solution in ferrite. Carbide-forming alloyed elements can be in various structural states: they can be dissolved in ferrite or cementite (FeCr) 3 C or exist in the form of independent structural components - special carbides: WC, MoC, etc. The location of carbide-forming elements in the steel structure depends on the amount of alloying elements introduced and the carbon content. Alloying elements dissolved in ferrite distort its crystal lattice; reduce the thermal and electrical conductivity of steel. The carbides of alloying elements are characterized by very high hardness (70-75 HRC) and wear resistance, but they have significant brittleness. They play a very important role in the production of tool steels. As studies have shown, a certain amount of alloying elements must correspond to a specific section of steel, otherwise its technological properties such as cutting, weldability, etc. worsen. brittleness temperature is the transition temperature of a metal from ductile to brittle fracture, and vice versa). 2. Theory of heat treatment The task of heat treatment is to cause an irreversible change in properties due to an irreversible change in the structure by heating and cooling. Any kind of heat treatment is usually depicted in temperature-time coordinates. Actually heat treatment does not provide for any other impact, except for temperature. During the heat treatment of steel, the following main transformations occur: 1) the transformation of pearlite into austenite, which occurs when heated above the Ac point1 : Fea + Fe3 C → Fev (C) or P - A;  Rice. 8. Heat treatment schedule: τн - heating time, τв - exposure time, τ0 - cooling time; t Max - Maximum temperature; teast is the true cooling rate at a given temperature, v = t Max - average cooling rate 2) the transformation of austenite into pearlite, which occurs during slow cooling from? - areas: Fev(C) → Fea(C) + Fe 3 C or A → P; 3) the transformation of austenite into martensite, which occurs during rapid cooling from? - areas: Fev (C) → Fea (C) or A → M; 4) transformation of martensite during heating (tempering): Fea (C) → Fea + Fe3 C or M → P. The description of the structural transformations that occur in steel during heat treatment is at the same time a theory of heat treatment. The transformation of pearlite to austenite is a necessary step in many heat treatments. 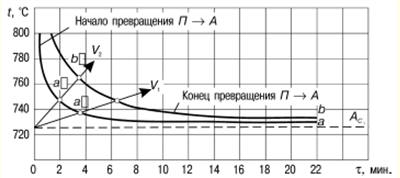 Rice. 9. Diagram of the isothermal transformation of pearlite (P) into austenite Steel with a carbon content (A) of 0,8%. The transformation of pearlite into austenite is realized when heated above the Ac value 1, and with increasing temperature it continuously accelerates. With continuous heating at different speeds, the rays v1 and v2 transformations begin at point a' (a') and end at point b' (b'), which is the higher, the greater the heating rate. In this regard, the faster the heating, the higher the heating temperature of the steel must be in order to cause the complete transformation of pearlite into austenite, including complete dissolution of carbides and homogenization of austenite. In the interval between the points a'b' (a "b"), the transformation proceeds at different rates, but approximately in the middle of the interval, the transformation proceeds with a strong absorption of heat so rapidly that a platform forms on the heating curve. This is usually the experimentally determined transformation temperature Ac1. With the initial pearlite structure, the formation of austenite comes from many centers, and immediately after the completion of the transformation of pearlite into austenite, fine-grained austenite is formed. Further heating leads to the growth of austenite grains, which is carried out according to one of the following mechanisms: by merging small grains into large ones, by migration of grain boundaries. The coalescence process takes place at a lower temperature (from +900 to +1000 °C) than migration (> +1100 °C), but leads to the formation of separate larger grains, i.e., inequigranularity. During heat treatment, the mechanical properties of steel can vary over a very wide range. So, for example, the hardness of steel containing 0,8% carbon, after such treatment, increases to 160-600 MV. 3. Diagram of the isothermal transformation of austenite On fig. 10 shows a diagram of the isothermal transformation of austenite in steel containing 0,8% carbon. Temperature is plotted along the y-axis. The abscissa is time.  Rice. 10. Diagram of the isothermal transformation of austenite in steel containing 0,8% carbon To study the isothermal transformation of austenite, small steel samples are heated to temperatures corresponding to the existence of stable austenite, i.e. above the critical point, and then quickly cooled, for example, to +700, +600, +500, +400, +300 ° C, etc. d., and kept at these temperatures until complete decomposition of the austenite. The isothermal transformation of austenite in eutectoid steel occurs in the temperature range from +727 to +250 ° C (temperature of the beginning of martensitic transformation - Mn). There are two C-shaped curves on the diagram. Curve I indicates the time of the beginning of the transformation, curve II - the time of the end of the transformation of supercooled austenite. The period before the start of the decomposition of austenite is called incubation. At +700 °C, the transformation of austenite begins at point a and ends at point b, resulting in the formation of pearlite. At a temperature of +650 °C, the decomposition of austenite occurs between points a1 and b1 . In this case, sorbite is formed - a thin (dispersed) mechanical mixture of ferrite and cementite. Steel dominated by the sorbitol structure has a hardness of 30-40 HRC. This steel has high strength and ductility. The stability of austenite largely depends on the degree of supercooling. Austenite has the least stability at temperatures close to +550 °C. For eutectoid steel, the stability time of austenite at temperatures from + 550 to + 560 ° C is about 1 s. As we move away from the temperature of +550 °C, the stability of austenite increases. The stability time at +700 °C is 10 s, and at +300 °C it is about 1 min. When steel is cooled to +550 °C (decomposition start and end points - a2 and b2 respectively - in the diagram) austenite turns into troostite - a mixture of ferrite and cementite, which differs from pearlite and sorbite in a high degree of dispersion of the components and has increased hardness (40-50 HRC), strength, moderate viscosity and plasticity. Below a temperature of +550 °C, as a result of the intermediate transformation of austenite (in the temperature range located below the pearlite, but above the martensite transformation), a bainite structure is formed, consisting of a mixture of carbon-saturated ferrite and carbides (cementite). Upon slow cooling, austenite transforms into pearlite, and at a high cooling rate, supercooled austenite completely transforms into sorbite. At even higher cooling rates, a new structure is formed - troostite. At the highest cooling rates, only martensite is formed, i.e., a supersaturated solid solution of carbon in? - iron. The cooling rate at which only martensite is formed from austenite is called the critical quenching rate. Austenite, which is retained in the steel structure at room temperature along with martensite, is called residual. Hardened high-alloy steels contain residual austenite in large quantities, while low-carbon steels have almost none. 4. Types and varieties of heat treatment: annealing, hardening, tempering, normalization Heat treatment of metals and alloys, as well as products made from them, is used to cause an irreversible change in properties due to an irreversible change in structure. Heat treatment is divided into the following types: proper thermal, chemical-thermal и deformation-thermal. Actually heat treatment does not provide for any other impact, except for temperature. If during heating the composition of the metal (alloy) - its surface layers - changes as a result of interaction with the environment, then such heat treatment is called chemical-thermal (CTO), and if, along with the temperature effect, deformation is also produced, making a corresponding contribution to the change in the structure, then such heat treatment is called deformation-thermal. In turn, deformation-heat treatment is divided into thermomechanical (TMT), mechano-thermal (MTO), etc. Different types of deformation-heat treatment are divided depending on the nature of phase transformations and the method of deformation. Actually heat treatment is divided into: annealing of the first and second kind, hardening with polymorphic transformation and hardening without polymorphic transformation, tempering and normalization. Annealing in general, it is a heat treatment process in which the metal is first heated to a certain temperature, maintained at this temperature for a specified time, and then slowly cooled, most often together with a furnace. Annealing of the first kind is the heating of a metal that has an unstable state as a result of a previous treatment (except for hardening), which brings the metal to a more stable state. Main subtypes: homogenization annealing, recrystallization annealing, stress relief annealing. Annealing of the second kind - heating above the transformation temperature, followed by slow cooling to obtain a stable structural state of the alloy. Hardening with polymorphic transformation - heating above the temperature of the polymorphic transformation, followed by sufficiently rapid cooling to obtain a structurally unstable state. Hardening without polymorphic transformation - heating to temperatures that cause structural changes (most often to dissolve the excess phase) followed by rapid cooling to obtain a structurally unstable state - a supersaturated solid solution. Vacation called a heat treatment process in which hardened steel is heated below the critical point Ac1, aged for a certain time, and then cooled. Normalization - one of the types of heat treatment During normalization, the steel is heated to temperatures 30-50 ° C higher than the upper critical temperatures, then held for the required time, and then cooled in still air to obtain a thin-lamellar pearlite structure. Normalization differs from annealing by faster cooling. 5. Surface hardening superficial such hardening is called, in which only a part of the surface layer of steel or alloy acquires high hardness. It differs from other hardening methods by heating. With this treatment, only the surface layer of the product is heated to the hardening temperature. Moreover, during rapid cooling, only this layer undergoes quenching. The rest is not hardened and retains the structure and properties that were before hardening. At present, surface hardening with induction heating by high-frequency currents is most widely used. This method of heat treatment creates the prerequisites for the comprehensive mechanization and automation of the hardening process. Induction heating of the metal is achieved by inducing eddy currents, which are concentrated in the surface layer of the product and heat it to a certain depth. The duration of heating by high-frequency currents is very short - it is calculated in seconds. When hardening small products produce heating and cooling of their entire surface. Hardening of products of considerable length is carried out by continuous-sequential heating. Water is used for cooling. For surface hardening of large products in single and small-scale production, as well as during repair work, heating with a flame is used, most often with oxyacetylene, the temperature of which is +3150 ° C. With this hardening method, the thickness of the hardened layer is 2-5 mm, its hardness is the same as in conventional hardening. In large-scale and mass production with a steady technological process, when the same products are made from steel of certain grades for a long time, for example, drive wheels of caterpillar tractors, surface hardening is used in an electrolyte - 14-16% aqueous solution of soda ash. The product to be hardened is connected to the negative pole of the DC generator and lowered into the electrolyte bath. The product immersed to a predetermined depth heats up in a few seconds, after which the current is turned off. As a rule, the same electrolyte is also the cooling medium. When heated in the electrolyte, electrolytic and electroerosive processes occur, which clean the heated surface of products from oxide films that impair heat transfer. The heating rate in the electrolyte is up to + 150 °C/s. There is also a method of pulse surface hardening. With it, high-frequency generators operating in a pulsed mode, capacitors, equipment for spot welding or laser installations are used. Such hardening makes it possible to eliminate deformations, cracks, increase the corrosion resistance of parts, and in some cases replace alloyed steel with carbon steel. In addition to the above methods of surface hardening, surface hardening in a fluidized medium is used. Fluidized medium ("fluidized" bed) is solid particles of quartz sand or other bulk material, intensively mixed with air or gas flow. The same medium is used for cooling. 6. Chemical-thermal treatment: carburizing, nitrocarburizing To change the chemical composition, structure and properties of the surface layer of parts, they are heat treated in a chemically active medium, called chemical-thermal treatment. With it, the following processes occur: the disintegration of molecules and the formation of atoms of a diffusing element (dissociation), the absorption of atoms by the surface (adsorption) and the penetration of atoms deep into the metal (diffusion). Cementation - diffusion saturation surface layer of the part with carbon. After carburizing, heat treatment is performed - hardening and low tempering. Such parts must have a hard hardened surface that is well resistant to abrasion, and a ductile core that can withstand dynamic loads. Parts made of steel containing up to 0,3% carbon are subject to hardening. The surface of parts is saturated with carbon in the range from 0,8 to 1% carburization, carried out in solid, liquid and gaseous media. In particular, a mixture of charcoal (60-90%) and barium carbonate salts (BaCO3) and sodium (NaCO3). When heated, the carbon in the charcoal combines with oxygen in the air to form carbon monoxide (CO), which decomposes to form atomic carbon that diffuses into the part: 2COCO2 + Catomic. With an increase in temperature and holding time, the thickness of the cemented layer increases, its depth reaches 0,5-2 mm for every 0,1 mm of the layer thickness, exposure is required for about 1 hour. In mass and large-scale production, good results are obtained by gas carburizing in special hermetically sealed furnaces . Compared to carburizing in a solid carburizer, gas carburizing makes it possible to increase the speed of the process, increase equipment throughput and labor productivity. After carburizing, the parts are subjected to heat treatment to ensure high surface hardness, correct the overheating structure, and eliminate the carbide network in the carburized layer. Hardening is carried out at a temperature of +780-850 °C, followed by tempering at +150-200 °C. Nitrocarburizing called the process of chemical-thermal treatment, in which there is a simultaneous saturation of the surface layers of steel products with carbon and nitrogen in a gaseous environment. After carbonitriding, the parts are hardened and then subjected to low tempering at a temperature of +160 to +180 °C. The hardness of the surface hardened and nitrocarburized layer is 60-62 HRC. Nitrocarburizing combines the processes of gas carburizing and nitrogen roving. The gas mixture includes endogas, up to 13% natural gas and up to 8% ammonia. Liquid carburetor - triethanolamine - is introduced into the working space of the shaft furnace in the form of drops. For alloy steels, the nitrocarburizing process is carried out in an atmosphere with a minimum amount of ammonia - up to 3%. 7. Chemical-thermal treatment: nitriding, ion nitriding Chemical-thermal treatment - nitriding is used to increase the surface hardness of various parts - gears, sleeves, shafts, etc. Nitriding - the last operation in the technological process of manufacturing parts. Before nitriding, complete thermal and mechanical treatment and even grinding are carried out; after nitriding, only finishing is allowed with metal removal up to 0,02 mm per side. Nitriding called chemical-thermal treatment, in which diffusion saturation of the surface layer with nitrogen occurs. As a result of nitriding, the following are provided: high hardness of the surface layer (up to 72 HRC), high fatigue strength, heat resistance, minimum deformation, high resistance to wear and corrosion. Nitriding is carried out at temperatures from +500 to +520 ° C for 8-9 hours. The depth of the nitrided layer is 0,1-0,8 mm. At the end of the nitriding process, the parts are cooled to + 200-300 ° C together with the furnace in an ammonia stream, and then in air. The surface layer is not susceptible to etching. Deeper than it is a sorbite-like structure. The process of liquid nitriding in molten cyanide salts is widely used in industry. The thickness of the nitrided layer is 0,15-0,5 mm. The nitrided layer is not prone to brittle fracture. The hardness of the nitrided layer of carbon steels - up to 350 HV, alloyed - up to 1100 HV. The disadvantages of the process are toxicity and high cost of cyanide salts. In a number of industries, ion nitriding is used, which has a number of advantages over gas and liquid nitriding. Ion nitriding is carried out in a sealed container in which a rarefied nitrogen-containing atmosphere is created. For this purpose, pure nitrogen, ammonia or a mixture of nitrogen and hydrogen are used. The parts placed inside the container are connected to the negative pole of a source of constant electromotive force. They act as a cathode. The anode is the body of the container. A high voltage (500-1000 V) is turned on between the anode and cathode - gas ionization occurs. The resulting positively charged nitrogen ions rush to the negative pole - the cathode. A high electric field strength is created near the cathode. The high kinetic energy possessed by nitrogen ions transforms into thermal energy. The part in a short time (15-30 minutes) is heated to +470 to +580 °C, nitrogen diffuses deep into the metal, i.e. nitriding. Compared to nitriding in furnaces, ion nitriding makes it possible to reduce the total duration of the process by 2-3 times, to reduce the deformation of parts due to uniform heating. Ion nitriding of corrosion-resistant steels and alloys is achieved without additional depassivating treatment. The thickness of the nitrided layer is 1 mm or more, the surface hardness is 500-1500 HV. Ion nitriding is applied to parts of pumps, injectors, lead screws of machine tools, shafts and much more. LECTURE No. 9. Classification of steels and their purpose 1. Carbon and alloy structural steels: purpose, heat treatment, properties High-quality carbon structural steels are used to produce rolled products, forgings, calibrated steel, silver steel, section steel, stampings and ingots. These steels are the main material for the manufacture of such machine parts as shafts, spindles, screws, nuts, stops, rods, hydraulic cylinders, chain sprockets, i.e., parts of various degrees of loading. Various special types of heat treatment of carbon steels are carried out in order to provide the necessary parameters of viscosity, elasticity and hardness. Ultimately, the heat treatment of these steels and parts leads to an increase in their wear resistance and reliability. High-quality carbon structural steels have higher mechanical properties than ordinary quality steels, due to the lower content of phosphorus, sulfur and other non-metallic inclusions in them. According to the types of processing, carbon structural steels are divided into hot-rolled, forged, calibrated and silver (with a special surface finish). Depending on the state of the material, these steels are produced without heat treatment, heat-treated (T) and hard-worked (H). In accordance with the purpose, hot-rolled and forged carbon structural steels are divided into subgroups: "a" - for hot forming; "b" - for machining by cutting on machine tools; "in" - for cold drawing. alloyed steels are called, which, in addition to the usual impurities (manganese, silicon, sulfur and phosphorus), contain a number of elements specially introduced into the steel during its smelting to obtain the desired properties. These elements are called alloying. Nickel, chromium, tungsten, molybdenum, titanium, vanadium, aluminum are most often used as alloying elements. Structural alloyed steels are divided into hot-rolled, forged, calibrated and silver steel, used in a heat-treated state. Hot-rolled and forged steels are supplied both in a heat-treated state (annealed, high-annealed, normalized or normalized with high tempering), and without heat treatment, calibrated and silver steel - hard-worked or heat-treated (annealed, tempered, normalized, hardened with tempering) . The standard (GOST) provides for the production and manufacture of 13 groups of structural alloyed steels, each of which was named according to the alloying element prevailing in it. For example, chromium alloy steels - 15X, 15Xa, 20X, 30X, 30XPA, 35X, 38XA, 40X, 45X, 50X; these steels are used to manufacture parts that, along with high wear resistance, require minimal deformation during heat treatment, improved and hardened parts operating at medium speeds and high specific pressures (gears, rings, gear racks, etc.), loaded parts of cars and tractors , as well as large parts requiring high hardenability and overall increased strength. 2. Steels resistant to corrosion Corrosion resistant steels - These are stainless steels and alloys that are resistant to electrochemical and chemical corrosion (atmospheric, soil, alkali, acid, salt), intergranular corrosion and stress corrosion. These steels include the following grades: 20X13 (2X13), 08X13 (0X13), 25X13H2 (2X14H2, EI474). They are used for the manufacture of parts with increased plasticity, subjected to shock loads (hydraulic press valves), parts operating in slightly aggressive environments (at atmospheric precipitation, in aqueous solutions of salts, organic acids); high corrosion resistance is ensured after heat treatment and polishing. Steel grade 14Kh14N12 (1Kh17N2, EI268) is mainly used in the chemical and aviation industries; possesses quite satisfactory technological properties. Steel grade 15X25T (X25T, EI439) is used in the production of heat exchange equipment (pipes, connecting flanges, valves, taps) operating in aggressive environments; used as a substitute for steel grade 12X18M10T in the manufacture of welded structures operating in more aggressive environments than those recommended for steel grade 08X17T; the use of this steel (15X25T) at temperatures of +400-700 °C is not recommended. 08X21N6M2T is used for the manufacture of parts and welded structures operating in environments of increased aggressiveness - acetic acid, sulphate and phosphate; grades 10Kh17N13M2T, 10Kh17N13M3T are used for the production of welded structures operating under the action of boiling phosphoric, sulfuric and 10% acetic acids, as well as in sulfuric acid environments. In a number of units of mechanisms, bearings operate in aggressive environments and at elevated temperatures. These assemblies mainly use 95×18 stainless steel. The microstructure of corrosion-resistant steel 95 × 18 is hidden acicular martensite and excess carbides, and the microstructure of a similar steel 11 × 18 M is hidden and finely crystalline martensite and excess carbides, but acicular martensite in steel 11 × 18 M is not allowed. In the case of bearings operating at temperatures from -200 °C to +120 °C, the best combination of mechanical and anti-corrosion properties of the steels used takes place in the following heat treatment mode: heating - up to +350 °C, final heating at +1070 °C ± 20 ° C, quenching in oil at a temperature of +30 to +60 °C, cold treatment - at -70 °C and tempering - from +150 to +160 °C. As long-term practice of application in various industries has shown, the corrosion resistance of steels depends on many factors: 1) from the alloying elements used - chromium, nickel, aluminum, titanium, molybdenum, their combinations and percentage in alloys; for example, chromium-molybdenum-new and chromium-molybdenum-vanadium steels of grades 15XM, 20XM, 30X3MF, 40XMFA have high anti-corrosion properties; 2) from thermal or chemical-thermal treatment; 3) on the quality of surface treatment of steels and parts operating in aggressive environments ("mirror" surfaces, as a rule, are more resistant to corrosion than rough ones). 3. Heat resistant steels and alloys Heat resistant steels and alloys belong to the third group of high-alloy steels. Their microstructure after heat treatment should consist of latent and finely acicular martensite or finely acicular martensite and excess carbides of alloying elements (MoC, CrC, NiC, etc.). Heat-resistant steels and alloys include: 1) 40X9C2. It is used for the manufacture of motor valves and fasteners operating at high temperatures - about +1000 ° C; 2) X1560-N. It is used for the manufacture of heating elements (the operating temperature of the heating elements is +1000-1300 °C); 3) Kh20N80, Kh20N80-VI (smelted by vacuum-induction method); 4) Kh15N60-N-VI, N50K10, Kh13Yu4, OH23Yu5, OH23Yu5A, Ox27Yu5A. These alloys are used to manufacture temperature sensors and temperature-sensitive elements, wire and tape for heating furnaces, electric thermal devices, microwire for non-critical resistors; these alloys operate in the range from +1000 to +1300 °C. To heat-resistant steels and alloys also includes the following brands: 1) KhN60Yu. It is used for the manufacture of turbine parts (from sheet metal) operating at moderate stresses, as well as for resistance heating devices; 2) 20X23H18. It is used for the manufacture of machine parts for the chemical and oil industries, shut-off valves for gas pipelines, combustion chambers, as well as for resistance heating devices; 3) 09X16N15M3B. It is used in the production of superheater pipes and high pressure pipelines; 4) 12X18H10T, 12X18H12T, 12X18H9T. They are used for the manufacture of parts of exhaust systems and pipes (from sheet and long products), steel 12X18H12T is more stable in operation than steel of the 12X18H10T brand; 5) 40X15N7G7F2MS. It is used for the manufacture of fasteners operating at a temperature of +650 °C. The heat resistance of steels and alloys depends on the composition of alloying elements, their combination and concentration. GOST 5632-72 recommends the optimal temperature ranges at which parts made of heat-resistant steels and alloys are most reliable in operation. In addition, the standard for each grade of steel or alloy indicates the temperature of the onset of intense scale formation and the service life of parts made of them - short-term, limited, long-term and very long. For a short period of operation, the service life of a part is conditionally taken up to 100 hours, limited - up to 1000 hours, long - up to 10 hours and very long - up to 000 hours. Heat-resistant alloys are highly alloyed and precision. Precision alloys are characterized by high purity of components, their exact ratio. The marking of precision alloys is slightly different from the marking of alloy steels and alloys. GOST 10994-74 regulates the chemical composition, basic physical properties and applications of each alloy. High-temperature precision alloys were listed above and their areas of application were indicated - N50K10, Kh13Yu4, OH23Yu5, Kh15N60-N, etc. 4. Tool materials: tool and high speed steels Tool alloy steels are used for the manufacture of cutting and measuring tools, as well as stamps. Steels intended for the manufacture of cutting tools (cutters, drills, cutters, etc.) must have high hardness (HRC l 62) and wear resistance. If cutting is performed under difficult conditions - high cutting speeds, machining of hard metals, a large section of the chip being removed - then significant mechanical energy is expended, which is accompanied by strong heating of the cutting edge of the tool. Therefore, the steel used for the manufacture of tools must have high hardness and heat resistance (or red hardness). The steels used for making dies must have a combination of hardness and toughness, as well as heat resistance (the ability to resist a sudden change in temperature in the form of resistance to the occurrence of fire cracks). Tool alloy steels contain carbide-forming elements: chromium, tungsten, molybdenum, manganese, vanadium. These steels have a slower cooling rate during hardening, thereby reducing the risk of cracking, deformation and warping. Steel is supplied hot-rolled, forged, calibrated and ground (silver). The standard provides for two groups and five subgroups of tool alloy steels. The content of both sulfur and phosphorus in them should not exceed 0,03%, and the sulfur content in steel obtained by electroslag remelting should not exceed 0,015%. Steels for cutting and measuring tools are made with shallow (7HF, 8HF 11HF) and deep hardenability (9X1, X, 12X1, 9XS, 8GS, 8X6NFT). Dies taps, drills, milling cutters, hacksaw blades, gauges, templates, etc. are made from these steels. High-speed tool steels got their name because the tools made from them can work at high cutting speeds without losing their properties. A remarkable property of high-speed steels is high red hardness, i.e., the ability to maintain high hardness and cutting ability when heated to 600-650 ° C. Red hardness is determined mainly by two factors: chemical composition and heat treatment. High speed steels have a complex chemical composition. Their most important alloying element is tungsten (6-18%), as well as vanadium (1-5%). In addition, all high-speed steels include chromium (3-4,5%), most of which dissolves in the iron crystal lattice. In order to give high-speed steels high cutting properties, they are subjected to heat treatment according to a special regime. The standard provides for the production of 14 grades of high-speed steels, which are conditionally divided into two groups: the first group - steels that do not contain cobalt, the second group - steels containing an increased amount of cobalt and vanadium. Grades of high-speed steels - R18, R12, R9, R6M3, R9K5. LECTURE No. 10. Hard and superhard alloys 1. Carbide and cutting ceramics Hard alloys and cutting ceramics are obtained using powder metallurgy methods. Powder metallurgy is a field of technology covering a set of methods for manufacturing metal powders from metal-like compounds, semi-finished products and products from them, as well as from their mixtures with non-metal powders without melting the main component. Raw materials for hard alloys and cermets - powders - are obtained by chemical or mechanical methods. Forming blanks (products) is carried out in a cold state or when heated. Cold shaping occurs during axial pressing on mechanical and hydraulic presses or under liquid pressure on an elastic shell into which powders are placed (hydrostatic method). By hot pressing in dies under a hammer (dynamic pressing) or by the gas-static method in special containers, due to the pressure (15-400 thousand Pa) of hot gases, products are obtained from poorly sintering materials - refractory compounds that are used for the manufacture of hard alloys and cermets. The composition of such sintered refractory compounds (pseudo-alloys) includes non-metallic components - graphite, alumina, carbides, which give them special properties. In the tool industry, hard sintered alloys and cutting cermets (metals + non-metal components) are widely used. According to the content of the main components powders in a mixture of hard sintered alloys are divided into three groups of tungsten, titanium-tungsten and titanium-tantalum-tungsten, by area of application - on alloys for processing materials by cutting, equipping mining tools, for surfacing fast-wearing machine parts, instruments and fixtures. Physical and mechanical properties of hard alloys: ultimate strength in bending - 1176-2156 MPa (120-220 KGS / mm 2), density - 9,5-15,3 g/cm 3, hardness - 79-92 HRA. Hard alloys for chipless metal processing, surfacing of quickly wearing parts of machines, instruments and devices: VK3, VK3-M, VK4, VK10-KS, VK20-KS, VK20K. In the designation of grades of hard alloys, the letter "K" means - cobalt, "B" - tungsten carbide, "T" - titanium and tantalum carbides; the figures correspond to the percentage of powders of the components included in the alloy. For example, VK3 alloy contains 3% cobalt, the rest is tungsten carbide. The shortage of tungsten necessitated the development of tungsten-free hard alloys that are not inferior in basic properties to sintered alloys based on tungsten carbides. Tungsten-free and chromium-carbide hard cermet alloys are used in mechanical engineering for the manufacture of drawing dies, drawing dies, for spraying various, including abrasive, materials, friction parts operating at temperatures up to 900 ° C, cutting tools for processing non-ferrous metals. 2. Superhard materials Three types of superhard materials (SHM) are currently used in various industries, including machine-building, for the manufacture of various cutting tools: natural diamonds, polycrystalline synthetic diamonds, and composites based on boron nitrite (elbor). Natural and synthetic diamonds have such unique properties as the highest hardness (HV 10 kgf/mm 2), they have very small: coefficient of linear expansion and coefficient of friction; high: thermal conductivity, adhesive resistance and wear resistance. The disadvantages of diamonds are low bending strength, brittleness and solubility in iron at relatively low temperatures (+750 °C), which prevents their use for machining iron-carbon steels and alloys at high cutting speeds, as well as with interrupted cutting and vibrations. Natural diamonds are used in the form of crystals fixed in the metal body of the cutter. Synthetic diamonds of the ASB (balas) and ASPK (carbonado) grades are similar in structure to natural diamonds. They have a polycrystalline structure and have higher strength characteristics. Natural and synthetic diamonds are widely used in the processing of copper, aluminum and magnesium alloys, noble metals (gold, silver), titanium and its alloys, non-metallic materials (plastics, textolite, fiberglass), as well as hard alloys and ceramics. Synthetic diamonds compared to natural ones, they have a number of advantages due to their higher strength and dynamic characteristics. They can be used not only for turning, but also for milling. Composite is a superhard material based on cubic boron nitride, used for the manufacture of blade cutting tools. In terms of hardness, the composite approaches diamond, significantly exceeds it in terms of heat resistance, and is more inert to ferrous metals. This determines its main area of application - the processing of hardened steels and cast irons. The industry produces the following main STM grades: composite 01 (elbor - R), composite 02 (belbor), composite 05 and 05I and composite 09 (PTNB - NK). Composites 01 and 02 have high hardness (HV 750 kgf/mm 2), but low bending strength (40-50 kg/mm 2). Their main area of application is fine and finishing shockless turning of parts made of hardened steels with a hardness of HRC 55-70, cast irons of any hardness and hard alloys of grades VK 15, VK 20 and VK 25 (HP ^ 88-90), with feed up to 0,15 mm /rev and cutting depth of 0,05-0,5 mm. Composites 01 and 02 can also be used for milling hardened steels and cast irons, despite the presence of impact loads, which is explained by more favorable milling dynamics. Composite 05 occupies a middle position in hardness between composite 01 and composite 10, and its strength is approximately the same as that of composite 01. Composites 09 and 10 have approximately the same bending strength (70-100 kgf / mm 2). 3. Materials of abrasive tools Abrasives divided into natural and artificial. The former include quartz, emery, corundum, and diamond, while the latter include electrocorundum, silicon carbide, boron carbide, cubic boron nitride, and synthetic diamonds. Quartz (P) is a material consisting mainly of crystalline silica (98,5 ... 99,5% SiO2). It is used for the manufacture of abrasive skins on a paper and fabric basis in the form of grinding grains in a free state. Emery (Н) - fine-crystalline alumina (25…60% Al2 O3) dark gray and black with an admixture of iron oxide and silicates. Designed for the manufacture of emery cloth and bars. Corundum (E and ESB) - a mineral consisting mainly of crystalline alumina (80.95% Al2 O3) and a small amount of other minerals, including those chemically related to Al2 O3. Corundum grains are hard and, when broken, form a conchoidal fracture with sharp edges. Natural corundum has a limited use and is used mainly in the form of powders and pastes for finishing operations (polishing). Diamond (A) is a mineral that is pure carbon. It has the highest hardness of all substances known in nature. Single-edged cutting tools and diamond-metal pencils for dressing grinding wheels are made from crystals and their fragments. There are four types of electrocorundum: 1) normal electrocorundum 1A, smelted from bauxites, its varieties - 12A, 13A, 14A, 15A, 16A; 2) white, smelted from alumina, its varieties - 22A, 23A, 24A, 25A; 3) alloyed electrocorundum smelted from alumina with various additives: chromium 3A with varieties 32A, 33A, 34A and titanium 3A with variety 37A; 4) A4 monocorundum, smelted from bauxite with iron sulfide and a reducing agent, followed by isolation of corundum single crystals. Electrocorundum consists of aluminum oxide Al 2 O 3 and some impurities. Silicon carbide - chemical compound of silicon with carbon (SiC). It has greater hardness and brittleness. than electrocorundum. Depending on the percentage of silicon carbide, this material comes in green (6C) and black (5C) colors. The first contains at least 97% silicon. The second type (black) is produced by the following varieties: 52C, 53C, 54C and 55C. Various abrasive tools (for example, grinding wheels) are made from green silicon carbide grains for processing hard alloys and non-metallic materials, and tools (grinding wheels) are made from black silicon carbide grains for processing products made of cast iron, non-ferrous metals and for sharpening cutting tools (cutters). , drills, etc.). Cubic boron nitride (KNB) - a compound of boron, silicon and carbon. CBN has a hardness and abrasive ability similar to diamond. Synthetic diamond (AS) has the same structure as natural. The physical and mechanical properties of synthetic diamonds of good grades are similar to those of natural diamonds. Synthetic diamonds are produced in five grades ASO, ACP, ASK, DIA, ACC. LECTURE No. 11. Non-ferrous metal alloys 1. Non-ferrous metals and alloys, their properties and purpose The valuable properties of non-ferrous metals have led to their widespread use in various branches of modern production. Copper, aluminum, zinc, magnesium, titanium and other metals and their alloys are indispensable materials for the instrument-making and electrical industries, aircraft and radio electronics, nuclear and space industries. Non-ferrous metals have a number of valuable properties: high thermal conductivity, very low density (aluminum and magnesium), very low melting point (tin, lead), high corrosion resistance (titanium, aluminum). Aluminum alloys with other alloying elements are widely used in various industries. Magnesium-based alloys are characterized by low density, high specific strength, and are well machined by cutting. They have found wide application in mechanical engineering and, in particular, in the aircraft industry. Technical copper, containing no more than 0,1% impurities, is used for various types of current conductors. Copper alloys According to their chemical composition, they are classified into brass and bronze. In its turn brass according to their chemical composition, they are divided into simple ones, alloyed only with zinc, and special ones, which, in addition to zinc, contain lead, tin, nickel, and manganese as alloying elements. Bronzes also subdivided into tin and tinless. Tinless bronzes have high strength, good anti-corrosion and anti-friction properties. Magnesium is widely used in metallurgy, with the help of which deoxidation and desulfurization of some metals and alloys, modify gray cast iron to obtain spherical graphite, produce metals that are difficult to recover (for example, titanium), mixtures of magnesium powder with oxidizers are used to make lighting and incendiary rockets in jet technology and pyrotechnics. The properties of magnesium are greatly improved by alloying. Aluminum and zinc with a mass fraction of up to 7% increase its mechanical properties, manganese improves its corrosion resistance and weldability, zirconium, introduced into the alloy together with zinc, refines the grain (in the alloy structure), increases mechanical properties and corrosion resistance. Molded castings are made from magnesium alloys, as well as semi-finished products - sheets, plates, rods, profiles, pipes, wires. Industrial magnesium is obtained electrolytically from magnesite, dolomite, carnallite, sea water and various production wastes according to the scheme for obtaining pure anhydrous magnesium salts, electrolysis of these salts in the molten state and refining magnesium. In nature, powerful accumulations form magnesium carbonates - magnesite and dolomite, as well as carnallites . In the food industry, packaging foil made of aluminum and its alloys is widely used - for wrapping confectionery and dairy products, and aluminum utensils are also used in large quantities (cookers, trays, bathtubs, etc.). 2. Copper alloys Copper is one of the metals known since ancient times. The early acquaintance of man with copper was facilitated by the fact that it occurs in nature in a free state in the form of nuggets, which sometimes reach considerable sizes. Currently, copper is widely used in electrical engineering, in the construction of power lines, for the manufacture of telegraph and telephone equipment, radio and television equipment. Copper is used to make wires, cables, tires and other conductive products. Copper has high electrical and thermal conductivity, toughness and corrosion resistance. Its physical properties are due to the structure. It has a cubic face-centered spatial lattice. Its melting point is +1083 °C, boiling point is +2360 °C. The average tensile strength depends on the type of processing and ranges from 220 to 420 MPa (22-45 kgf / mm 2), relative elongation - 4-60%, hardness - 35-130 HB, density - 8,94 g/cm 3. Possessing remarkable properties, copper, at the same time as a structural material, does not meet the requirements of mechanical engineering, therefore it is alloyed, that is, metals such as zinc, tin, aluminum, nickel and others are introduced into alloys, thereby improving its mechanical and technological properties. In its pure form, copper is used to a limited extent, more widely - its alloys. According to the chemical composition, copper alloys are divided into brass, bronze and copper-nickel, according to the technological purpose - into deformable, used for the production of semi-finished products (wire, sheet, strip, profile), and foundry, used for casting parts. Brass - alloys of copper with zinc and other components. Brasses containing, in addition to zinc, other alloying elements are called complex or special, and are named according to the alloying components introduced, in addition to zinc. For example: tompak L90 is brass containing 90% copper, the rest is zinc; aluminum brass LA77-2 - 77% copper, 2% aluminum, the rest is zinc, etc. Compared to copper, brass has great strength, corrosion resistance and elasticity. They are processed by casting, pressure and cutting. Semi-finished products are made from them (sheets, tapes, strips, pipes of condensers and heat exchangers, wire, stampings, valves - taps, valves, medals and badges, art products, musical instruments, bellows, bearings). Bronzes are copper-based alloys in which tin, aluminum, beryllium, silicon, lead, chromium and other elements are used as additives. Bronzes are subdivided into tin-free (BrA9Mts2L, etc.), tin (BrO3ts12S5, etc.), aluminum (BrA5, BrA7, etc.), silicon (BrKN1-3, BrKMts3-1), manganese (BrMts5), beryllium bronzes (BrB2, BrFNT1,7, etc.). Bronzes are used for the production of stop valves (faucets, valves), various parts operating in water, oil, steam, slightly aggressive media, sea water. 3. Aluminum alloys The name "aluminum" comes from the Latin word alumen - so for 500 years BC. e. called aluminum alum, which was used for etching when dyeing fabrics and tanning leather. In terms of prevalence in nature, aluminum ranks third after oxygen and silicon and first among metals. In terms of use in technology, it ranks second after iron. Aluminum does not occur in free form, it is obtained from minerals - bauxites, nephelines and alunites, while alumina is first produced, and then aluminum is obtained from alumina by electrolysis. The mechanical properties of aluminum are low: tensile strength - 50-90 MPa (5-9 kgf / mm 2), elongation - 25-45%, hardness - 13-28 HB. Aluminum is well welded, but difficult to machine, has a large linear shrinkage - 1,8% In its pure form, aluminum is rarely used, mainly its alloys with copper, magnesium, silicon, iron, etc. are widely used. Aluminum and its alloys are necessary for aviation and mechanical engineering, power lines, metro rolling stock and railways. Aluminum alloys are divided into cast and wrought. Cast aluminum alloys are produced in ingots - refined and unrefined. Alloys, in the designation of grades of which there is the letter "P", are intended for the manufacture of food utensils. The mechanical properties of alloys depend on their chemical composition and production methods. The chemical composition of the main components included in the alloy can be determined by the grade. For example, AK12 alloy contains 12% silicon, the rest is aluminum; AK7M2P - 7% silicon, 2% copper, the rest is aluminum. The most widely used in various industries is an alloy of aluminum with silicon - silumin, which is produced in four grades - SIL-00, STR-0, STR-1 and STR-2. In addition to aluminum (base) and silicon (10-13%), this alloy includes: iron - 0,2-0,7%, manganese - 0,05-0,5%, calcium - 0,7-0,2 %, titanium - 0,05-0,2%, copper - 0,03% and zinc - 0,08%. Various parts for cars, tractors, passenger cars are made from silumins. Aluminum wrought alloys in ingots, intended for pressure treatment and for hemming in the production of other aluminum alloys, are standardized by certain standards. Alloys for pressure treatment consist of aluminum (base), alloying elements (copper - 5%, magnesium - 0,1-2,8%, manganese - 0,1-0,7%, silicon - 0,8-2,2 %, zinc - 2-6,5% and a small amount of other impurities). The brands of these alloys: VD1, AVD1, AVD1-1, AKM, semi-finished products are made from aluminum alloys - sheets, strips, strips, plates, ingots, slabs. In addition, non-ferrous metallurgy produces aluminum antifriction alloys used for the manufacture of monometallic and bimetallic bearings by casting. Depending on the chemical composition, the standard provides for the following grades of these alloys: AO3-7, AO9-2, AO6-1, AO9-1, AO20-1, AMST. The standard also defines the operating conditions for products made from these alloys: load from 19,5 to 39,2 MN / m2 (200-400 kgf / cm 2), temperature from 100 to 120 °C, hardness - from 200 to 320 HB. 4. Titanium alloys Titanium - Silvery white metal. It is one of the most common elements in nature. Among other elements in terms of prevalence in the earth's crust (0,61%), it ranks tenth. Titanium is light (its density is 4,5 g/cm 3), refractory (melting point 1665 ° C), very strong and ductile. A resistant oxide film forms on its surface, due to which it resists corrosion well in fresh and sea water, as well as in some acids. At temperatures up to 882 °C, it has a hexagonal close-packed lattice; at higher temperatures, it has a body-centered cube. The mechanical properties of sheet titanium depend on the chemical composition and the method of heat treatment. Its tensile strength is 300-1200 MPa (30-120 KGS / mm 2), elongation - 4-10%. Harmful impurities of titanium are nitrogen, carbon, oxygen and hydrogen. They reduce its ductility and weldability, increase hardness and strength, and worsen corrosion resistance. At temperatures above 500 °C, titanium and its alloys readily oxidize by absorbing hydrogen, which causes embrittlement (hydrogen embrittlement). When heated above 800 °C, titanium vigorously absorbs oxygen, nitrogen and hydrogen; this ability is used in metallurgy to deoxidize steel. It serves as an alloying element for other non-ferrous metals and for steel. Due to their remarkable properties, titanium and its alloys are widely used in aircraft, rocket and shipbuilding. Semi-finished products are made from titanium and its alloys: sheets, pipes, rods and wire. The main industrial materials for producing titanium are ilmenite, rutile, perovskite and sphene (titanite). The technology for producing titanium is complex, time-consuming and time-consuming: first, a titanium sponge is produced, and then malleable titanium is produced from it by remelting in vacuum furnaces. sponge titanium, obtained by the magnesium-thermal method, serves as the starting material for the production of titanium alloys and other purposes. Depending on the chemical composition and mechanical properties, the following grades of spongy titanium are established as standard: TG-90, TG-100, TG-110, TG-120, TG-130. In the designation of brands, the letters "TG" mean - sponge titanium, "Tv" - hard, the numbers mean Brinell hardness. Sponge titanium includes impurities: iron - up to 0,2%, silicon - up to 0,04%, nickel - up to 0,05%, carbon - up to 0,05%, chlorine - up to 0,12%, nitrogen - up to 0,04 0,1%, oxygen - up to 1%. For the manufacture of various semi-finished products (sheets, pipes, rods, wire), titanium and titanium alloys processed by pressure are intended. Depending on the chemical composition, the standard provides for the following grades: VT00-1, VT0-4, OT0-4, OT1-4, OT5, VT5, VT1-6, VT20, VT22, VT7, PT-7M, PT-1V, PT -0,2 m. Main components: aluminum - 0,7-0,2%, manganese - 2-0,5%, molybdenum - 5,5-0,8%, vanadium - 5,5-0,8%, zirconium - 3-0,5%, chromium - 2,3-2%, tin - 3-0,15%, silicon - 0,40-0,2%, iron - 1,5-XNUMX%. Iron, silicon and zirconium, depending on the grade of the alloy, can be the main components or impurities. 5. Zinc alloys Zinc-copper alloy - brass - was known to the ancient Greeks and Egyptians. But the smelting of zinc on an industrial scale began only in the XNUMXth century. Zinc - metal of light gray-bluish color, brittle at room temperature and at 200 °C, when heated to 100-150 °C it becomes ductile. In accordance with the standard, zinc is produced and supplied in the form of ingots and blocks weighing up to 25 kg. The standard also establishes zinc grades and their areas of application: TsV00 (zinc content - 99,997%) - for scientific purposes, obtaining chemical reagents, manufacturing products for the electrical industry; CVO (zinc - 99,995%) - for the printing and automotive industries; TsV1, TsV (zinc - 99,99%) - for the production of pressure castings intended for the manufacture of parts for especially critical purposes, to obtain zinc oxide, zinc powder and pure reagents; ZOA (zinc 99,98%), ZO (zinc 99,975%) - for the manufacture of zinc sheets, zinc alloys processed by pressure, white, ligatures, for hot and galvanic galvanizing; Ts1S, Ts1, Ts2S, Ts2, Ts3S, Ts3 - for various purposes. Zinc alloys are widely used in industry: brass, zinc bronze, alloys for coating various steel products, for the manufacture of galvanic cells, printing, etc. Zinc alloys in ingots for casting are standardized. These alloys are used in automotive and instrumentation, as well as in other industries. The standard establishes the grades of alloys, their chemical composition, the products made from them are determined: 1) TsAM4-10 - especially critical parts; 2) TsAM4-1 - critical parts; 3) TsAM4-1V - non-critical parts; 4) TsA4O - critical parts with stable dimensions; 5) CA4 - non-critical parts with stable dimensions. Zinc anti-friction alloys, intended for the production of monometallic and bimetallic products, as well as semi-finished products, by casting and pressure treatment are normalized by the standard. The mechanical properties of alloys depend on their chemical composition: tensile strength δВ = 250-350 MPa (25-35 KGS/mm 2), relative elongation δ = 0,4-10%, hardness - 85-100 HB. The standard establishes the grades of these alloys, their areas of application and working conditions: TsAM9-1,5L - casting of monometallic liners, bushings and sliders; allowable: load - 10 MPa (100 kgf / cm 2), sliding speed - 8 m/s, temperature 80 °C; if bimetallic parts are obtained by casting in the presence of a metal frame, then the load, sliding speed and temperature can be increased up to 20 MPa (200 KGS / cm 2), 10 m/s and 100 °C, respectively: TsAM9-1,5 - obtaining a bimetallic tape (zinc alloy with steel and duralumin) by rolling, the tape is intended for the manufacture of liners by stamping; allowable: load - up to 25 MPa (250 kgf / cm 2), sliding speed - up to 15 m/s, temperature 100 °C; AM10-5L - casting of bearings and bushings, allowable: load - 10 MPa (100 KGS/cm 2), sliding speed - 8 m/s, temperature 80 °C. LECTURE No. 12. Properties of non-metallic materials 1. Non-metallic materials Back in the second half of the XNUMXth century. in our country, much attention was paid to the use of non-metallic materials in various industries and the national economy as a whole. The production of various non-metallic materials was established and constantly increased: synthetic resins and plastics, synthetic rubbers replacing natural rubber, high-quality polymers with specified technical characteristics, including reinforced and filled plastics. Plastics and other non-metallic materials have a number of excellent physical, chemical, mechanical and technological properties, which led to their widespread use in various industries - mechanical engineering, electrical engineering, electronics, etc. As a structural material, plastics are increasingly replacing expensive metals. The use of plastics makes it possible to constantly improve designs. Equipping machines and equipment, as well as partial assembly of various units, can reduce their weight, improve reliability and durability, and increase productivity. The production of plastics requires 2-3 times less capital investments than the production of non-ferrous metals. The starting materials for the production of plastics are cheap products of processing of coal, oil and natural gas. Plastics are reinforced to improve mechanical properties. For the manufacture of various parts operating in friction (sliding) mechanisms with low loads and speeds, non-metallic materials such as anti-friction polymer and plastic materials are used. These materials have a low coefficient of friction, high wear resistance, chemical resistance, and can operate without lubrication. However, low thermal conductivity, significant (tens of times greater than that of metals) coefficient of thermal expansion, low hardness and high compliance limit the possibility of their wide use. They are most effectively used in combination with other materials, metals and plastics. In addition, brake woven asbestos tapes and friction asbestos linings are used as friction non-metallic materials - molded, pressed, woven, cardboard-bakelite and spiral-wound, which can be used in all climatic zones. Friction asbestos linings are used for friction units of automobiles, aircraft, tractors, metal-cutting and textile machines, handling equipment and diesel locomotives. The resource of such non-metallic linings operating in friction units is quite high. For example, for cars with diesel engines, it is 6000 hours, cars - 125 km, trucks - 000 km. Brake woven asbestos tapes are used as linings in brake and friction units of machines and mechanisms with surface friction temperature up to 75 °C. Non-metallic materials are widely used in various industries and the economy as a whole. 2. Polymers: structure, polymerization and polycondensation, properties At present, it is difficult to imagine medicine without polymer systems for blood transfusion, medical equipment - without transparent polymer tubes, patient care items - without rubber heating pads, ice packs, etc. Significantly enrich the range of materials used in medicine, synthetic polymers. Polymers differ significantly from metals and alloys: their molecules are elongated into long chains, as a result of which polymers have a high molecular weight. Polymer molecules are obtained from the initial low molecular weight products - monomers - by polymerization and polycondensation. Polycondensation polymers include phenol-formaldehyde resins, polyesters, polyurethanes, and epoxy resins. Polyvinyl chloride, polyethylene, polystyrene, polypropylene are high-molecular compounds of the polymerization type. High-polymer and high-molecular compounds are the basis of organic nature - animal and plant cells, consisting of protein. For the manufacture of many medical devices, both polymeric materials, which are based on natural raw materials, and artificial materials - synthetic and polymeric materials - are widely used. Most dressings are made from polymeric materials of natural origin: cotton wool, gauze and products made from them, alignin, as well as suture threads (surgical silk). Polymers are the basis of plastics used in the manufacture of various instruments, parts of medical equipment and equipment. Polymers such as phenol-formaldehyde liquid and solid resins have found wide application in various industries and the economy as a whole. Phenol-formaldehyde liquid resol type resins - a product of the polycondensation of phenol and formaldehyde in the presence of a catalyst with or without the addition of modifying and stabilizing agents - are supplied as a homogeneous transparent liquid from reddish-brown to dark cherry color with an average density of 1,2 g/cm 3. They are used in the production of heat and sound insulating products, plywood, chipboard and wood fiber boards, abrasive tools on a flexible basis, fiberglass, asbestos and asbestos friction products, carbon fiber for mine roofing, etc. Grades of resins: SFZh-303, SFZh-305, etc. Solid phenol-formaldehyde resins of novolac and resol types - products of polycondensation of phenols (or their fractions) and formaldehyde in the presence of a catalyst with or without the addition of modifying substances. Available in the form of powder, flakes and crumbs. They are used to produce rubber compounds, molding masses, laminated plastics, varnish conductive suspensions, anticorrosive paints and varnishes and adhesives, as binders for abrasive products and shell molds, in the manufacture of foam plastic, in the production of oil varnishes for the paint and varnish and food industries. The following grades of resins are produced: SF-010A, SF-010, SF-010M (modified), SF-014, etc. 3. Plastics: thermoplastic, thermoset, gas-filled Plastics - plastics - these are materials obtained on the basis of a high-molecular organic compound - a polymer that acts as a binder and determines the main technical properties of the material. Depending on the elasticity, plastics are divided into three groups: rigid, elastic modulus 700 MPa, up to 70 MPa Plastics are produced monolithic in the form thermoplastic and thermosetting and gas-filled - cellular structure. Thermoplastic plastics include low-pressure polyethylene, polypropylene, high-impact polystyrene, ABS plastics, polyvinyl chloride, fiberglass, polyamides, etc. Thermosetting plastics include: rigid polyurethane foams, aminoplasts, etc. К gas-filled plastics include polyurethane foams - gas-filled ultralight structural material. thermoplastic - low-pressure polyethylene - an ethylene polymerization product obtained at low pressure using complex organometallic catalysts. The base grades of this polyethylene are: 20108-001, 20208-002, 20308-005, etc. The density of the polyethylene is from 0,931 to 0,970 g/cm 3. High impact polystyrene is a product of copolymerization of styrene with rubber or other plasticizer, which has higher mechanical properties than general purpose polystyrene. It has high hardness, impact strength, elasticity, tensile strength, temperature resistance in the range from +65 to -40 °C. Aminoplasts - thermosetting plastics - pressing urea- and melamine-formaldehyde masses obtained on the basis of amino resins using fillers (organic, mineral or combinations thereof), coloring and modifying substances. Their Marten heat resistance is at least 100-180 ° C, impact strength - 3,9-29,4 kJ / m 2 (4-30 kgf × cm/cm 2), shrinkage - 0,2-0,8%, specific volumetric electrical resistance - 1? 10 11 -1×10 12 Ohm × cm. From aminoplasts, products for household, technical and electrical purposes are manufactured by hot pressing. In total, 11 grades of aminoplasts are produced: KFA-1, KFB-1, etc. Polyurethane foams - gas-filled plastics - ultralight structural material. Polyethers and polyesters, isocyanates, catalysts and emulsifiers are the starting materials for their production. Elastic polyurethane foams (PPU) have closed, non-communicating gas-filled cells (foam plastics) and communicating cells (foam plastics). The general term "foams" is often used. Elastic foam contains 70% air communicating pores. It has a density of 25-29 kg/m 3, well resists decay, substances used in the dry cleaning of products, its tensile strength is 0,07-0,11 MPa. Elastic polyurethane foam is used in the production of upholstered furniture, car seats, tractors and other products. Rigid polyurethane foam is used for the manufacture of chair bodies, decorative elements, as heat and sound insulating materials. Filled foam plastics (PPU) have become widespread in recent years. 4. Elastomers The term "elastomers" was introduced to replace the names "synthetic rubbers" and also "natural rubber". Elastomers polymers are called polymers that have high elasticity in a wide temperature range - the ability to undergo significant (from several hundred to 1000% or more) reversible deformations at relatively small acting loads. The first elastic material of this kind was natural rubber, which has not lost its importance in the production of elastomers, including for medical products, due to its non-toxicity. Rubber is obtained from latex (the milky juice of Brazilian hevea), consisting of more than half of water, in which 34-37% rubber, 2-2,7% protein, 1,65-3,4% resin, 1,5-4,92% are dissolved. .50% sugar. In plantations where natural rubber is prepared as an industrial raw material, latex is coagulated with organic acids, rolled into corrugated sheets and smoked in smoke chambers at a temperature of +2,5 °C. The constituent substances of smoke play the role of antiseptics and stabilizers of rubber oxidation. Such sheets with a thickness of 3-5 mm with a wafer surface pattern are called "smoketsheet". They serve as the most commonly used form of raw plantation rubber. Elemental analysis data for purified rubber correspond to the empirical formula C8HXNUMX (isoprene). Synthetic rubbers (elastomers) are obtained by polymerization from monomers with the participation of catalysts (process accelerators). The first Soviet synthetic rubber was obtained by S. D. Lebedev from technical alcohol. Currently, several types of synthetic rubbers (elastomers) are produced, including isoprene, which differs little from natural. For medical products, saloxane (silicone) rubber is used, the main polymer chain of which consists of silicon and oxygen atoms. It is heat-resistant and physiologically inert. The raw materials for the manufacture of synthetic rubbers are oil, natural gas, and coal. The transformation of rubber or a "raw" rubber mixture into elastic rubber (a material with the necessary performance properties) is carried out by vulcanization. Vulcanization, like heat treatment of metals and alloys, leads to a change in the structure of rubber. During vulcanization, elastomer molecules are joined (“crosslinked”) by chemical bonds into a spatial three-dimensional network, as a result of which a material is obtained that has the necessary elastic and strength properties (strength, elasticity, hardness, tear resistance, etc.). The main vulcanizing agent is sulfur; tellurium and selenium are also used. The more sulfur is added to the rubber, the harder and less elastic the elastomer becomes. In modern production, in addition to vulcanizers, organic accelerators are widely used, the presence of which reduces the amount of sulfur (up to 2% instead of 10%) and the vulcanization temperature. There are ultra-accelerators, thanks to which vulcanization proceeds at room temperature instead of a temperature of +130-150 °C. 5. Rubber Rubbers of various types and brands belong to the group of elastic materials - elastomers. Rubbers are divided into shaped and non-shaped. Non-moulded rubber includes a large group of so-called raw rubbers. Raw rubbers are produced under numbers (10, 11, 14, etc.) in the form of plates of different thickness, coated with talc (to prevent sticking), or in the form of rolls with a fabric gasket (from calico), which also protects the rubber from adhesion. Unshaped raw rubber is obtained by vulcanization from rubber compounds made on the basis of synthetic rubbers or natural. The main vulcanizing agent is sulfur, but selenium and tellurium are also used. Depending on the brands, raw rubber is used to obtain various molded products with certain properties. For example, several types of technical sheet rubber are obtained from raw rubber: acid-alkali, heat-resistant, frost-resistant, food-grade, etc. Frost-resistant rubber retains its properties at temperatures down to -45 ° C. Technical sheet rubber with a thickness of 3-4 mm is used for the manufacture of sealing gaskets in flanged joints of pipelines transporting cold water, and rubber with a fabric gasket (made of synthetic fabric) is also used for transporting hot water with temperatures up to +100 ° C. Various rubber products are obtained from raw rubbers - couplings, rings, valves, various gaskets, etc., using the following molding methods: pressing, extrusion and injection molding. The process of pressing rubber products takes place in vulcanizing hydraulic presses under a pressure of 100-300 atm. and at a temperature of +140-160 °C. In the production of upholstered furniture, foam rubber is widely used, which is a material based on synthetic or natural rubber. For the manufacture of foam rubber, a latex mixture is used, which is kept for 18-21 hours, foamed and vulcanized, followed by drying. Foam rubber is produced in the form of sheets or molded furniture elements. In terms of elasticity, elasticity, residual deformation, foam rubber is an ideal material for upholstered furniture. Foam rubber self-ventilating and cooled by passing air through communicating pores. To reduce the weight of foam furniture elements, they are made with voids, but in order to maintain the ability to withstand significant loads, the volume of voids should not exceed 40% of the volume of the entire element. Rubbers intended for the manufacture of individual groups of products are subject to additional requirements that ensure that the products fulfill their functional purpose and are reliable in operation. Currently, the industry produces sheet rubber of three grades: heat-frost-acid-alkali-resistant (TMKShch); limited oil and petrol resistant (OMB); increased oil and petrol resistance (PMB), which, in turn, are subdivided according to the hardness of the rubber used: soft (M) for operation at temperatures from -45 ° C to +90 ° C; medium hardness (C) - at temperatures from -60 °C to +80 °C, increased hardness (P) - at temperatures from -60 °C to +80 °C. 6. Sealants Sealants (sealants) are used almost everywhere - in construction, in the housing and communal services system, mechanical engineering, furniture production, in everyday life, during various repair work. Sealants are polymer compositions in the form of pastes, putties or liquids, which, after being applied to the surface, thicken immediately or after some time as a result of the vulcanization of the polymer base. For the preparation of sealants, liquid synthetic rubbers and special additives are used. The industry produces various types of sealants: building facade, suture-thiokol and acrylate, building rubber-silicone, acrylic. In glass work, thiokol sealants 7-30M and UT-31 are mainly used to seal joints, which are vulcanized at temperatures from +18 °C to +30 °C. In the housing and communal services system, KLT-30 silicone sealant is widely used to seal threaded connections operating in the temperature range from -60 °C to +200 °C. In recent years, many brands of sealants produced by foreign companies have been imported to Russia: DAP, KVADRO, KIMTEC, KRASS. Compared with other similar materials, sealants are moisture resistant, gas-tight, and durable. Sealants based on polyisobutylene are used to seal external joints between elements of prefabricated large-panel buildings. Sealants, like rubber, belong to the group of elastomers. The most widely used thiokol sealants, which are characterized by versatility. The Russian industry produces the following brands of thiokol sealants: 1) U-30M. Supplied complete as part of the U-30 black sealant paste, vulcanizer No. 9 and vulcanization accelerator - diphenylguanidine, mixed immediately before use in a ratio of 100: 7: 0,35 mass parts. Designed for sealing metal (except brass, copper, silver) and other joints operating in dilute acids and alkalis, liquid fuels and in air in all climatic conditions at temperatures from -60 °C to + 130 °C; 2) UT-31 - light gray paste U-31, vulcanizer No. 9 and vulcanization accelerator, used for sealing metal (except brass, copper, silver) and other compounds operating in air and liquid fuels at temperatures from -60 °C up to +130 °C and up to + 150 °C - briefly in air; 3) 51-UT-36A (with adhesive) and 51-UT-36B (without adhesive) - dark gray putty paste U-36, epoxy resin E-40 (for 51-UT-36B) and two-chrome sodium as a vulcanizer ; used in instrumentation. For sealing various joints, seams operating at temperatures from +200 °C to +300 °C, heat-resistant siloxane sealants made on the basis of liquid siloxane rubbers are intended. Brands of siloxane-new sealants are as follows: elastosil 11-01, silpen. VPT-2L, KL-4, KLT-30, KLSE, VGO-2, KLVAE, etc. Heat and fuel-resistant sealants are also produced based on fluorine-containing rubbers of the following grades: VGF-1, VGF-2, 51-G-1 and others LECTURE No. 13. Glass. Decorative materials 1. Glass: inorganic and organic In various industries, construction and other sectors of the economy, inorganic and organic glasses are used. inorganic glass subdivided into technical, construction and domestic. In turn, building glass is divided into structural, finishing, sound and heat insulating. Surface quality glass is polished and unpolished, colored and colorless. According to the method of hardening - ordinary, annealed, hardened and hardened by chemical or other means. According to the profile, glass is produced flat, wavy, bent and profiled. Glass inorganic construction has found wide application in construction: for glazing light openings in walls, lanterns (in the roofs of various buildings). Inorganic glass is obtained by cooling a melt containing pure quartz sand (silica), sodium sulfate and limestone. The greatest application for glazing of window and door blocks, partitions was received by sheet window glass of 1 and 2 grades. The density of this glass is 2000-2600 kg/m 3, light transmission - 84-87%, low thermal conductivity. The industry also produces sheet patterned glass of grades 1 and 2, colorless and colored with a relief pattern; thermally polished sheet glass, colored sheet glass (red, blue, green, yellow), smooth, colored and colorless; with a smooth, corrugated or patterned surface; unreinforced and reinforced with steel mesh (3 types are available: channel profile; box-shaped profile - with one or two seams; ribbed profile); sheet glass reinforced with metal mesh - colorless and colored, smooth and corrugated, patterned. Organic glass - product of unsaturated polyester resins, transparent polymer. It is divided into technical, structural, sheet, lighting and watch. Technical organic glass is a plasticized and non-plasticized polymer (copolymer) of methacrylic acid methyl ester, widely used in various industries and economy in general. The standard provides for three grades of TOSP glass - plasticized technical organic glass; TOSN - non-plasticized technical organic glass; TOSS - technical organic copolymer glass. Physical and mechanical properties of technical organic glass: softening point (depending on thickness) - 92-130 °C, impact strength - 6-9 kJ/m 2 (6-9 kgf - density at 20 ° C), transparency (with a thickness of up to 30 mm) - 85-88%, overheating shrinkage at 40 ° C for 1 hour - 3,5-4%, tensile stress at break - 60-80 MPa (600-800 kgf/cm 2), relative elongation at break - 2-2,5%. Structural organic glass is available in three grades: SOL - plasticized organic glass; ST-1 - organic non-plasticized glass and 2-55 - copolymer glass. These grades of organic glass are used as a structural material in the instrument and assembly industry. 2. Sitalls, metal glasses Glass-ceramics (glass ceramics) - glass-ceramic materials based on glass, which differ from the latter in a crystalline structure similar to ceramic, but with smaller (from fractions to 1-2 microns) crystals and their denser packing, excluding any material porosity. Glass-ceramics are produced by melting a glass charge of special compositions with the addition of crystallization, cooling the melt to a plastic state and molding products from it using glass technology methods (pressing, blowing, drawing). Molded products are subjected to special heat treatment to form a fine-grained dense structure, characteristic of glass-ceramics. Glass-ceramics are subdivided into the following groups according to their chemical composition: STL - spodumene; STM - cordierite; STB - boron-barium and boron-lead, high-silicon, photositals. Sitals of the STL brand contain lithium, STM brands - magnesium. Sitalls can be transparent, opaque, white, cream and colored. By properties glass-ceramics are divided into: chemically resistant, wear-resistant, optical, electrically insulating and heat-resistant. Chemically resistant and wear-resistant glass-ceramics are used for the manufacture of chimneys, plungers, parts of chemical pumps, reactors and chemical equipment, where high heat resistance and gas-liquid impermeability are required. In the manufacture of synthetic fibers, wear-resistant glass-ceramics are used for thread wires and some other parts of textile machines; in addition, they are used to make instruments for measuring the lengths and angles of various products. Optical glass-ceramics with TCLE (thermal resistance) close to zero are used primarily for the manufacture of astronomical mirrors and lasers. Electrical insulating glass-ceramics due to their electrical properties, especially at high temperatures, they are used for the manufacture of radio and electronic devices and installations, various devices operating in conditions of variable temperature and humidity, as well as insulators operating in high voltage mode. Heat-resistant glass-ceramics with TLCR close to zero are used as structural materials for devices operating under variable thermal loads, as well as in the production of heat exchangers. metal glass have the same structure as that of the Si-talls, only the coating is metallic. Certain metal compounds are added to the basic composition during the production of such glasses (which depend on the purpose and field of application of metallic glasses), from which, at a given temperature in a special atmosphere (melting medium), a metal coating is released on the surface of the glass mass. Metal glasses are used primarily in electrical engineering. Metallic glasses are also produced by hot spraying on a glass-ceramic material (for example, applying a layer of aluminum 0,5-1 mm thick). Such a coating withstands a rapid change in temperature, despite the significant difference in the TLC of aluminum and glass-ceramic material. 3. Polymorphic modifications of carbon and boron nitride It is widely used in various branches of industry and, above all, in mechanical engineering. cubic boron nitrile (CBN) - crystalline cubic modification of the boron compound with nitrogen, synthesized according to the technology inherent in the production of synthetic diamonds. Due to the variation of technological factors, various types of cubic boron nitride are produced - elbor, elbor-R, cubo-nit, ismite, hexanite, etc. Cubic boron nitride and its varieties are measured by carats, their classification by grain size is also close to the standards adopted for steel processing and iron-based alloys. In recent years, CBN polycrystals up to 12 mm in size have been obtained. Widely used in mechanical engineering are superhard materials obtained on the basis of boron nitride - elbor-R and ismit. In terms of cutting properties and wear resistance, they are several times superior to cermet hard alloys and mineral ceramics. CBN-R cutters are made of two types: prefabricated, in which CBN blanks are mounted in a transition insert installed in the cutter body, and solid, where blanks (ELBOR-R) are attached directly to the tool body by pouring them with liquid (molten) metal. The use of Elbor-R allows to ensure high productivity and cleanliness of the treated surface. The most effective use of Elbor-R is when turning hardened steels instead of grinding and when boring holes. The superhard material ismite, obtained on the basis of boron nitride (modification), has a higher resistance than hard alloys when turning hardened steels. The cubic crystalline modification of carbon is diamonds - natural and synthetic, which are insoluble in acids and alkalis, have high hardness, are used for the manufacture of cutters, glass cutters, tips for measuring the hardness of metals, etc. 4. Composite materials In various sectors of the country's economy, including construction, various composite materials based on chopped wood are widely used: chipboard, fibreboard, wood concrete, fiberboard, cement-bonded chipboard and wood-adhesive compositions. Chipboards are made by hot pressing wood particles mixed with a binder. Such plates are widely used in construction, in furniture production. Dimensions of plates: length ranging from 1830 mm to 5680 mm, width - from 1220 mm to 2500 mm, thickness - from 8 mm to 28 mm. According to physical and mechanical parameters, chipboards are divided into grades: P-A and P-B - according to the quality of the surface with a regular and fine-grained surface; according to the degree of surface treatment - polished and unpolished; according to hydrophobic properties - with normal and increased water resistance; have one drawback - low tensile strength perpendicular to the layers. Fiberboard are made using waste from the processing of coniferous and hardwood wood. Depending on the density and bending strength of the board, wood-fiber boards are classified into soft (M-4, M-12, M-20), semi-solid (PT-100), hard (T-350, T-400), superhard - ( ST-500). According to their technical properties, they are made bio-, fire-, moisture-resistant and sound-absorbing. Soft fiberboard is used in construction as a material for thermal and sound insulation of walls, partitions, ceilings, interfloor ceilings, etc. Semi-hard fiberboard is used for cladding walls and ceilings in residential and public buildings. Hard and super hard fiberboards are widely used in furniture production (for the rear walls of cabinet furniture, the lower parts of drawers, etc.), in construction - for cladding walls, ceilings, etc. Such boards are produced with a thickness of 2,5-10 mm. Medium-hard fiberboards are produced in large volumes abroad under the brand name "MDF boards - Medium Density Firebrands" with a thickness of 10 to 30 mm, for the manufacture of modern furniture as a substitute for plywood and natural wood. In recent years, various products from wood concrete have been widely used in construction, which is made using crushed woodworking waste, a binder - Portland cement, additives - calcium chloride, liquid glass, aluminum sulphate and lime. Arbolite It is used for the production of wall panels, various heat-insulating products. As enclosing structures in the construction of wooden houses, farms and various buildings in rural areas, cement boards, which are made using wood shavings, Portland cement and chemical additives. Plates are produced in the following sizes: 1200? 3600 mm, thickness 8-25 mm; their density is within 1100-1400 kg/m 3, flexural strength - 9-12 MPa. For the manufacture of molded containers are widely used wood adhesive compositions, consisting of crushed wood and a binder - urea-formaldehyde resins with an additive - paraffin. 5. Synthetic facing materials In the last decade, a variety of synthetic facing materials have been widely used to decorate the interiors of offices, various premises and outdoor work, which have replaced scarce sliced veneer. Moreover, they have greatly simplified the finishing technology, especially such facing materials as decorative films based on adhesive and polymer materials (in ). Currently, the technology of obtaining film materials with imitation of "real" pores is used. Such a film of the PDSO and PDO brands (without an adhesive layer) is used for furniture veneering, interior decoration of cars. The film PDO-A-020 is used in the aviation industry for finishing aircraft cabins. Films based on polymeric materials are made from compositions of polyvinyl chloride, polypropylene, polyester, etc. The above films PDO and PDSO are polyvinyl chloride (imported too). Recently, polyvinyl fluoride films (PVF), which have good performance properties, have been used for veneering various wood products (door panels, furniture), as well as walls and panels, interior elements. For these purposes, in addition to the PVF film, self-adhesive films based on a copolymer of vinyl chloride and vinyl acetate grade VA, which are manufactured by Skoch, are used. These films are produced transparent, colored, with a metallization effect. In great demand among various consumers are protective adhesive tapes polymer-based types LT-38, LT-50, which are used to protect the edging material from drips of paintwork material when finishing boards. Adhesive tapes are a polymer base - a film 35-50 microns thick, on which a thin sticky layer is applied. In the manufacture of countertops, window sills, doors, sanitary equipment, laminates (a kind of synthetic facing materials) are often used. La minates are heat-strengthened laminates obtained by pressing paper at high temperature. The paper base of the laminate is impregnated with phenolic resin, and the outer layers are impregnated with melamine. Laminates are wear resistant, food compatible, easy to clean, non-flammable, moisture resistant. For upholstery of furniture, finishing of various types of transport are widely used faux leather: upholstery vinyl artificial leather, upholstery porous-monolithic vinyl leather, upholstery porous-monolithic artificial leather, etc. Artificial leathers are also in great demand among shoe manufacturers. In recent years, new materials have been used to decorate residential and public interiors - very original, with a variety of designs, artificial stones, which are mineral acrylic boards. They are hard, like natural stones, have a different structure, are resistant to abrasion, and are relatively easy to process. Porous monolithic films are also used for facing the facade surfaces of furniture for bedrooms, cabinets, and children's furniture, having an upper monolithic surface and a lower porous layer (its thickness is 1,2-1,5 mm, width - 600-1360 mm, roll length - 30- 50 m). 6. Decorative laminates Decorative Laminates have been used for many years for finishing residential, public and industrial premises, interiors of various vehicles, for lining the working surfaces of kitchen, medical and commercial furniture. Plastics of this type have good physical-mechanical and decorative properties, are well processed, resistant to high temperatures, to impact and abrasion, to the action of water, steam, as well as food and household liquids (tea, coffee, vodka, ethyl alcohol, etc.). d.). The density of DBS plastics is not less than 1,4 g/cm 3, breaking stress in tension - not less than 63,6 MPa, in bending - 98 MPa (for grade A - 17,6 MPa), water absorption is not more than 4%, heat resistance - from + 120 to + 140 ° C. DBS plastics are divided into grades A, B, C - depending on the quality of the front surface and physical and mechanical parameters. Grade A plastic is used in operating conditions that require increased wear resistance, for example, for table tops. Grade B plastic is used under less severe operating conditions - for finishing vertical surfaces. Grade B plastic is used as an ornamental material. Decorative Laminates (DBSP) are sheets of compressed paper impregnated with thermosetting resins. In the manufacture of DBSP, a protective layer impregnated with melamine-formaldehyde resin is applied to a decorative layer of paper (one-color or with a pattern). For the manufacture of a protective film, highly refined cellulose from hardwood or cotton is used. DBSP is produced in one-color, various color printed patterns that imitate precious wood, stone, marble, fabric, leather, etc. By purpose, these plastics are divided into structural, facing and molded. Structural DBSP have a thickness of more than 1 mm, are used in various designs. Facing plastics more elastic and have a thickness of up to 1 mm, are used as a finishing material. According to the operating conditions, the surfaces of furniture and other types of boards facing chipboard are divided into two main groups. Group I - working and front surfaces of commercial and other furniture that are directly exposed to the external environment; Group II DBS plastics are used on the front surfaces of kitchen, children's and other furniture products that are not constantly exposed to moisture, heat and other factors. Molded DBSP under the action of heat and pressure can change their shape. They are used for wrapping shaped parts with complex rounded shapes or corners. One solid sheet of formable plastic DBS is lined with the face and edge of the part - this technology is called postforming. DBS plastics are produced with a length of 400-3000 mm, a width of 400-1600 mm and a thickness of 1,0; 1,3; 1,6; 2,0; 2,5 and 3,0 mm. Reverse side of 1,0 thick plastic; 1,3 and 1,6 mm should be rough. For gluing DBS plastics, various adhesives are used - PVA, bustilat, epoxy, as well as KN-2 mastics. LECTURE No. 14. Insulating materials 1. Classification of thermal insulation materials During the construction of industrial facilities, civil structures, the accompanying communications of heat and water supply are protected from the effects of negative temperatures with the help of various types of heat-insulating materials. Divide thermal insulation materials into: 1) construction; 2) polymeric. Construction thermal insulation materials by structure there are: 1) fibrous; 2) cellular; 3) granular. And depending on feedstock: 1) inorganic (foam glass, lightweight concrete with fillers, mineral wool); 2) organic (foam plastics, honeycomb plastics, fibrolite, wood-fiber and peat slabs, etc.); 3) polymeric. On shape and appearance thermal insulation materials are divided into: 1) piece (slabs, half-cylinders, blocks, lightweight bricks, etc.); 2) rolled and corded (plaits, mats, cords); 3) loose and loose (glass and mineral wool, perlite sand, etc.). On hardness thermal insulation materials are divided into: 1) hard, increased rigidity; 2) rigid; 3) semi-rigid; 4) soft. On thermal conductivity they are divided into three classes: 1) A - low thermal conductivity; 2) B - medium; 3) B - increased. The main indicator of thermal insulation materials is the coefficient of thermal conductivity, which for most of them is in the range of 0,02-0,2 W / m? °C. On flammability thermal insulation materials are produced: 1) fireproof; 2) slow-burning; 3) combustible. Polymer thermal insulation materials are divided into: 1) rigid, with a compressive strength of 5 squeeze = 0,15 MPa; 2) semi-rigid; 3) elastic with 5 squeeze = 0,01 MPa. Polymer heat-insulating materials for construction purposes are durable, have a wide range of deformation characteristics, chemically and water resistant. 2. Types of heat and sound insulation materials For thermal insulation of pipelines with a diameter of 15-25 mm and the corresponding shut-off valves, canvas-stitched fabric from waste glass fibers of the KhPS-T-5,0 and KhPS-T-2,5 grades is widely used, it is designed for a maximum temperature of +450 ° C, has an average density 400-500 kg/m 3, thermal conductivity - 0,053 W/(m × °С), designed for temperatures up to + 300 °C, flame retardant. Mats made of glass staple fiber on a synthetic binder brand MT-35 are designed for thermal insulation of pipelines with a diameter of 57 to 426 mm, have an average density of 60 kg/m 3, thermal conductivity 0,047 W/(m × °C), maximum application temperature +180 °C, flame retardant. Heat-insulating cord made of mineral wool grade 200 is used to insulate pipelines with a diameter of up to 108 mm inclusive and shut-off valves, respectively, has a density of 220 kg / m 3, thermal conductivity 0,056 W/(m × °С), maximum application temperature from +150 °C to +600 °C, in a fiberglass sheath fireproof, in other cases - flame retardant. In recent years, heat-insulating materials made of URSA glass staple fiber have been widely used in Russia. URSA products are used in the construction of all types of buildings, for the insulation of equipment and pipelines, vehicles. Produced in the form of rolls, plates with a density of 13-75 kg/m 3 and mats with a density of 10-25 kg/m 3, 40-140 mm thick. At present, Russian-made heat-insulating material penophile is in great demand among various consumers. This material is composed of polyethylene foam and polished aluminum foil coating, has low thermal conductivity, high resistance to water vapor diffusion; used for insulation of walls, floors, for insulation of pipelines, tanks and valves in water supply and heating systems, etc. The Russian JSC "Kineks" produces extruded polystyrene "foam-plex" using Italian technology - polystyrene with a closed homogeneous cellular structure. In terms of thermal insulation properties, this material surpasses expanded clay concrete and foam concrete by 5-10 times, glass wool and mineral fiber boards by 2-3 times, has a density of 30 to 45 kg / m 3, the slabs have a width of 600 mm and a length of 1 to 4,5 m and a thickness of 30 to 100 mm; it is used for thermal insulation of roofs, floors, basements of residential and public buildings, swimming pools, etc. For sound insulation, elastic polyvinyl chloride foams of PVC-E, vinylopor, D, M and C grades are used, which have open cellular porosity. Semi-rigid polystyrene foam and vinipor PZh are used for the manufacture of profile products with sound-absorbing properties. Soundproofing materials are also: PE-2 foam, PE-5 and PE-7 foams; they are also used for thermal insulation. Sound-absorbing and sound-proof building materials and products can be the same materials that are used for thermal insulation: glass wool, mineral wool, foam plastics of various types and brands. 3. Waterproofing materials In construction, the housing and communal services system, various waterproofing materials are widely used, which are designed to protect building structures, buildings and structures from the harmful effects of water and chemically aggressive liquids - alkalis, acids, etc. By appointment waterproofing materials are divided into anti-filtration, anti-corrosion (metal), paint and varnish, glass enamels, oxide films, rubber, plastic and bituminous lubricants and sealing (pastes, putties or solutions). Waterproofing materials according to the type of base material are: asphalt (bitumen, asphalt mastic), mineral (cements, magnesia binders, dolomite, lime-nepheline binders, etc.) and metal. The following waterproofing materials are widely used in the construction and housing and public utilities systems: film (polyethylene, polypropylene and others, in particular "PIL" - insulating film with a sticky layer), towed and in the form of plates (polyisobutylene, rubber), mastic (bitumen, polyisobutylene) and rolled (glassine, roofing felt, roofing material). Mastic and rolled waterproofing materials are made on an artificial basis and on the basis of natural materials, tow and film - only on a polymer basis. A good waterproofing material based on organic binders are bitumen. Natural bitumen is a black substance, odorless, softens at a temperature of + 35-90 ° C, and hardens again when cooled. Artificial bitumen is obtained by distillation of natural bitumen (residual tar) or from waste oil refining (reclaimed tar). On the basis of bitumen, RB mastic (rubber bitumen) is prepared, which is a good waterproofing material. Before applying waterproofing coatings on walls, foundations are waterproofed with cement mortars (using sulfate-resistant cement) with the addition of ceresite, liquid glass, sodium aluminate. The greatest application in the performance of waterproofing of various building structures was found film polymeric materials, which produce four grades: "T" - for waterproofing during the construction of temporary structures, protective shelters; "B" and "B" 1"- for use in waterproofing reclamation and water facilities; "M" - for technical waterproofing. Waterproofing polyethylene films are produced with a thickness of 0,015-0,5 mm, a width of 800-6000 mm, a length of more than 50 m, a density of 910-929 kg / m 3. Builders are in great demand for polyvinyl chloride films for general purposes ("OH" grades) and for waterproofing (grades "P") Special films for waterproofing grade "P" have the following characteristics: thickness 0,03-0,27 mm, width - 15 g /m 2, water absorption - 0,5%; tensile strength - 8-19 MPa. When performing roof waterproofing, as a rule, according to the technology, waterproofing materials are used in the complex: bitumen, rubber-bitumen mastics, ruberoid films of the brand "P", hydroisol. 4. Electrical insulating materials In the context of the high prevalence of various electrical installations in almost all industries and the economy of the country as a whole, electrical insulating materials have been widely used. The most important characteristic of electrical insulating materials is their high electrical resistance. Electrical insulating materials subdivided into: gaseous (air, various gases); liquid (various oils and organosilicon liquids) and solid - of organic origin (resins, plastics, paraffins, waxes, bitumens, wood) and inorganic (mica, glass, ceramics, etc.). Such an electrical insulating material as mica belongs to the group of rock-forming minerals, the so-called sheet aluminosilicates. Mica, as an electrical insulating material, is divided into two types: phlogopite density - 2700-2850 kg / m 3 and hardness, on a mineralogical scale 2-3 and biotite-density - 2700-3100 kg / m 3, hardness, on a mineralogical scale 2,5-3. The most widespread electrical insulating materials created by organic synthesis. These materials are characterized by predetermined electrical, physicochemical and mechanical properties. The electrical insulating materials include fluoroplast-4, a product of the polymerization of tetrafluoroethylene, which is produced in the form of a white, easily clumping powder or plates. Fluoroplast-4, depending on the purpose, is divided into the following grades: "P" - for the manufacture of electrical insulating and capacitor films; "PN" - for the production of electrical products with increased reliability. For the manufacture of various electrical products are often used cast polyamide copolymers grades AK-93/7, AK-85/15 and AK-80/20 - products of joint polycondensation of "AG" salt and caprolactam. Polyamide cast copolymers have a dielectric constant at 10 6 Hz after a 24-hour stay in distilled water 4-5, and the specific surface electrical resistance (in the initial state) is 1 × 10 14 -1×10 15 Ohm × cm For many years, cast polyamide 610, a product of polycondensation of a salt of hexamethylenediamine and sebacic acid, has been used for the manufacture of electrical insulating products. Products are obtained by injection molding using polyamide 610 in the form of white and light yellow granules 3-5 mm in size. Polyamide 610 has the following characteristics: specific volume electrical resistance - not less than 1 × 10 14 Ohm × cm, electrical strength - not less than 20 kV/mm. Electrical insulating materials include aminoplasts used for several decades - pressing urea- and melamine-formaldehyde masses obtained on the basis of amino resins (thermosetting condensation products of formaldehyde with carbamide, melamine or their combination) using fillers (organic, mineral or combinations thereof). Aminoplastics are produced in several grades MFB - lighting, MFV - with increased electrical insulating properties, which have a specific volume electrical resistance of 1 × 10 11 -1×10 12 Ohm × cm 5. Lubricants In accordance with the standard, lubricants are classified by origin, physical state, presence of additives, purpose, and application temperature. By origin or raw material lubricants are divided into: 1) mineral lubricants, which are obtained by mixing hydrocarbons of mineral origin in their natural state or as a result of their processing; 2) petroleum lubricants - refined oil obtained on the basis of petroleum raw materials; 3) synthetic lubricants - materials obtained by synthesis; 4) vegetable lubricants - materials of plant origin; 5) animal lubricants obtained from raw materials of animal origin. By physical condition Lubricants are divided into gaseous, liquid, plastic and solid. By appointment lubricants are divided into: 1) motor, designed for internal combustion engines (carburetor, diesel, aviation, etc.); 2) transmission, used in transmissions of tractors, cars, self-propelled and other machines; 3) industrial, intended mainly for machine tools; 4) hydraulic, used in the hydraulic systems of various machines; 5) special - compressor, instrument, cylindrical, electrical insulating, vacuum, etc. According to application temperature among the above lubricants, there are: low-temperature (for units with a temperature not higher than +60 ° C) - instrumental, industrial and the like; medium temperature, used at temperatures from +150 to +200 ° C, - turbine, compressor, cylinder and the like; high-temperature, used in units that are exposed to temperatures up to +300 ° C and more. Currently, the main lubricants are mineral oils and lubricants derived from petroleum feedstocks, greases and cutting fluids. The main functions that lubricants must perform when used in assembly units of mechanisms, engines of various machines: reduce wear of rubbing surfaces of parts; to reduce the force of friction between the mating surfaces to help reduce unproductive energy losses; prevent the breakthrough of the working mixture and combustion products into the engine crankcase, i.e. improve the compression of the cylinder-piston group, etc. All mineral oils according to the method of production and composition divided into four groups: distillate, residual, blended and additive oils. The domestic industry produces the following motor oils: for diesel engines - M-8-V 2, M-8-G 2, M-8-G 2 K and so on; for carburetor engines - M-8-A, M-8-B, M-12-G 1 etc. In recent years, many imported motor oils have appeared in retail trade: ESSO, TEBOIL, MOBIL, CASTROL, etc. The Russian industry produces various greases: anti-friction (solid oil, lithol); multipurpose; high-temperature (CIATIM-221S, PFMS-4S, etc.), low-temperature (CIATIM-201, ZhRO, UNIOL-3M, etc.) and a number of other special applications. 6. Types of roofing materials The materials used for roofing in buildings of various types are divided into: rolled (roofing material, roofing felt, glassine, etc.), piece, or sheet (tiles, tiles, slate, etc.), and mastic (bituminous, tar, rubber - "RBC" and polymeric mastics). According to the type of raw materials, roofing materials are divided into organic - roofing felt, roofing felt, wood roofing tiles, tes, etc. and metal - galvanized and non-galvanized roofing steel. By type of constituent components (binders or binders) - on bituminous (roofing material, glass roofing material, glassine), tar (only roofing), polymer - mastics rubber-bitumen, bitumen-polymer, polymer, etc. In recent years, various types of flat and corrugated boards, tiles and sheets; rolled, synthetic materials, including those based on polyisobutylene, polyethylene, epoxy and phenolic resins. In addition, new effective roofing and waterproofing bitumen and bitumen-polymer materials of the built-up type on non-decaying bases are currently being used. New bitumen-polymer materials on strong and elastic bases include: isoplast, bicroplast, dneproflex, rubemast, filisol, etc. The advantage of these materials is that they are coated on both sides with a bitumen-polymer binder consisting of bitumen , polymer additives and filler. Till now as a roofing material in rural, settlement and partly in city construction the tile made of baked clay (clay tile) or from cement and sand solutions of a rigid consistence (cement tile) is used. Such tiles are durable and fire-resistant, but fragile and heavy, as they have a high density. In recent years, roofing metal tiles, which are produced by the Finnish company RANNILA STEEL, have been used as roofing material. These roof tiles are made of 0,5 mm hot-dip galvanized steel with a color polymer coating that withstands sunlight and temperature fluctuations. At present, a new original roofing material has appeared - bituminous tiles, which are produced by the Belarusian company Poleznaya Kompaniya TM. This tile is designed to cover pitched roofs, made of oxidized bitumen reinforced with fiberglass. Another Belarusian novelty is a polymer-concrete tile, which is absolutely waterproof, in terms of durability and frost resistance corresponds to at least 50 years of operation. When laying rolled roofing materials, polymer and bitumen-polymer cold mastics are used: MBK grades - butyl rubber based on butyl rubber; brand BLK - bitumen-latex - based on shale bitumen products. The use of the listed mastics simplifies the process of installing a roof with waterproofing. 7. Facing materials and their application In modern construction, a wide variety of facing materials are widely used to improve the operational and decorative qualities of buildings and various structures. Facing materials are made from ceramics, plastics, glass, natural stone, asbestos cement and special mortars. In the past twentieth century The most common facing materials were glass and ceramic tiles, slabs of shell rock, marble, granite and volcanic tuff. At the beginning of the XXI century. appeared and began to be widely used as a facing material plastic panels on a plastic (PVC) basis. These panels are used in residential premises and offices, for finishing rooms with high humidity. Such panels have many advantages: durability; do not deform; have 100% moisture resistance, do not require special care and are easy to clean; made from environmentally friendly materials. Currently in great demand among various consumers are polyvinyl chloride cladding embossed sheets, designed for finishing walls and ceilings in the premises of public and industrial buildings (except for children's and medical institutions). These sheets are made of four types: 1) single-layer single-color; 2) single layer multicolor; 3) two-layer one-color; 4) two-layer multi-color. All types of sheets have a length of 300 to 2000 mm, a width of 300-1000 mm, a thickness of 0,4-2 mm; various relief drawings, with a smooth or embossed front surface. In recent years, for interior decoration of walls and suspended ceilings of buildings with a relative humidity of not more than 60%, they have become widely used. decorative slabs of phosphogypsum, which are made from a gypsum binder obtained by autoclave processing of phosphogypsum. At the end of the twentieth century. began to produce a very original finishing material - glass wallpaper with a water-repellent and sound-absorbing effect, which are implemented by the Alaksar company (Moscow). This wallpaper is durable, easy to clean, does not fade, has 20 kinds of beautiful patterns; they have been used for several years in Sweden and are in high demand. Great popularity in Europe and Russia won stretch ceilings, suspended ceilings from various materials - film, fiberglass, mineral wool boards, polystyrene, aluminum panels. Stretch film ceilings used in the decoration of apartments, offices, bars, restaurants, swimming pools, etc. Fiberglass ceilings they have good sound absorption, while the echo effect is reduced, so they are used for finishing large rooms - meeting rooms, sports, shopping, etc. The most popular finishing material - wallpaper various types - foamed, vinyl, silkscreen, duplex and plain wallpaper - paper. New in recent years - thin plates vitreous glaze with a multi-color pattern and a self-adhesive base - used for wall cladding. This material was called "onliglas", produced by the Spanish company "Tres Estilos". LECTURE No. 15. Adhesives 1. Classification of adhesives and requirements for them In various sectors of the economy, various adhesive materials are widely used, which are made on the basis of natural (natural) or synthetic adhesives. natural adhesives are subdivided into adhesives of animal, vegetable and mineral origin. The starting materials for adhesives of animal origin are: tissues, bones, blood and milk of animals. Glutinous, casein, albumin glues are obtained from the indicated raw materials. Raw materials for adhesives of plant origin are: legume seed protein, starch, natural resins, rubber, dextrin. Adhesives mineral - silicate, asphalt, bituminous. Synthetic resins are the raw material for the production of synthetic adhesives. Synthetic adhesives are solutions of natural modified or synthetic polymers in water or alcohol. By reactivity, adhesives are divided into thermosetting, thermoplastic and dispersion adhesives. In turn, thermosetting adhesives are divided into: melamine, epoxy, resole, polyurethane, polyester, urea-formaldehyde, phenol-formaldehyde. К thermoplastic adhesives include: skin, bone, hot melt adhesives, nitrocellulose, polyvinyl acetate, polyvinyl chloride, etc. Rubber adhesives allocated to an independent class of adhesive materials. These include latex and rubber adhesives. Adhesives are widely used in furniture production, in the manufacture of shoes and in construction. In construction, adhesives are used for fixing various finishing materials, for building structures. Various adhesives are used in the aviation and automotive industries, in the decoration of passenger railway cars and subways. Adhesives are single-component, supplied ready-made, and multi-component, which are prepared mainly at the point of consumption (in particular, epoxy glue). Adhesive materials are divided depending on the materials to be glued: shoe - for gluing leather, rubber, leather substitutes; for bonding metals and non-metals; thermal insulation fabrics and gluing them to other materials; polymers, for gluing wood, in the manufacture of plywood, etc. All adhesives are subject to the following requirements: ensuring high strength of adhesive joints; high stability and viability during storage; high moisture, water resistance; non-toxicity; retention of mechanical strength over time. In furniture production, the strength of the adhesive joint is determined by testing the adhesive joint during chipping. In accordance with the specifications for the manufacture of furniture, adhesive materials must provide a shear strength along the adhesive layer in a dry state when facing at least 1 MPa, in other cases - at least 2 MPa. Water resistance of adhesives - the most important requirement for almost all adhesives. According to this indicator, adhesives are divided into waterproof, increased water resistance, limited water resistance and non-water resistant. Waterproof adhesives are mainly synthetic, limitedly waterproof - casein, non-waterproof - glutinous. 2. Synthetic thermoset adhesives Synthetic thermoset adhesives are cured by polycondensation or polymerization reactions at relatively high temperatures (up to +100 °C) in most cases. In the woodworking industry and furniture production, urea-formaldehyde hot-gluing adhesives of the following grades are widely used: KF-Zh in furniture production; similar adhesives KF-B (curing at +100 °C for 25-40 s), imported carbamide-doformaldehyde adhesive "Kleiberit 871" produced by the German company Kleiberit hot pressing for gluing plywood and facing faces. Hot curing adhesives include adhesives of the following brands: VK-32-EM, D-15, D-23, D-43, which are used for gluing metals and glass packs. In furniture production and construction, synthetic thermosetting adhesives are used, such as phenol-formaldehyde и resorcinol formaldehyde. These adhesives are used in cold or warm curing mode with a heating temperature of +60-80 °C. These include adhesives of the brands SFZh, FR-12, FR-100, DFK-1AM, etc. The listed adhesives are used in furniture production when gluing wood with metals and plastics, in construction in the manufacture of doors, window blocks, etc .; their curing time at a temperature of +20 ° C - from 5 to 25 hours. Widely used in various industries phenol polyvinyl acetate adhesives BF-2, BF-4, BF-6: BF-2 and BF-4 glue wood, polystyrene, metals, glass, ceramics. In great demand among different consumers are phenolic epoxy adhesives grades FE-10 and FR-10, which are used for gluing metals, various plastics and other materials in structures operating at temperatures up to +250 °C. High bonding strength, moisture and chemical resistance provide epoxy adhesives made on the basis of dianova resins, ED-20, ED-22, ED-16 and E-40; as well as adhesives of grades K-160, K-176 based on modified epoxy resin, which are used for bonding plastics; gluing wooden and plastic elements on lacquered surfaces. On the basis of modified epoxy resin, adhesives PED, PED-6 are made, which are used for gluing wood with plastics, fastening polyvinyl chloride plastic to the surface of building structures made of metal and reinforced concrete. Russian industry produces high quality polyurethane adhesives grades PU-2, PU-2M, PU-UV, VK-5 VK-11, which are used for gluing glass, ceramics, wood, metals, reinforced plastics, various polymeric materials. The German company Kleiberit produces PU-501 adhesive, which is in high demand due to its maximum efficiency in bonding mineral building boards, ceramic materials, layered bonding of wood, etc. The same company produces a two-component polyurethane adhesive PU for membrane pressing, while it has increased heat resistance, moisture and steam resistance. 3. Synthetic thermoplastic adhesives As well as thermosetting adhesives, in various sectors of the economy, including construction and furniture production, synthetic thermoplastic adhesives are widely used, which are used in the form of dispersions, solutions and hot melt adhesives: when gluing expanded polystyrene, wooden parts with polyvinyl chloride foam; in the production of all types of finishing works. The difference between thermoplastic adhesives and thermoset adhesives is that they retain the linear structure of macromolecule chains in the adhesive system, and bonding is carried out without chemical reactions. Thermoplastic adhesives are divided into polyvinyl acetate, hot melt adhesives, polyvinyl chloride, methinol polyamide, polymethyl methacrylate, nitrocellulose The disadvantage of these adhesives is their low heat resistance - at a temperature of +40 ° C they begin to soften, and at + 60-70 ° C the strength of the adhesive joint decreases sharply . Polyvinyl acetate adhesive in the form of dispersions is available under the following brands: PVA, D 50 N, D 5 ° C, D 50 V, etc. The most common PVA glue, which is used in furniture production and everyday life for gluing film finishing materials to various surfaces, glues wooden products, paper, cardboard, glass, porcelain, leather, etc. For many years, such synthetic glue as CMC - sodium carboxymethyl cellulose technical, sodium salt of cellulose glycolic acid, obtained by reacting alkali cellulose with sodium monochloroacetate or monochloroacetic acid, has been used in large volumes in construction and everyday life, i.e. CMC - a product of chemical processing of wood pulp. The advantages of CMC used as a binding material are as follows: it mixes well with pigments, does not change their color, as well as with starch, dextrin; emulsifies drying oil and some varnishes; has biological stability (almost does not rot). In recent years, various high-performance synthetic thermoplastic adhesives manufactured by the German company IGeiberit have been used in furniture production and construction, of the following brands: "Kleiberit 303" - based on polyvinyl acetate dispersion, designed for gluing (hot and cold) hard and tropical wood, laminated boards , spiked joints; mounting adhesive "Kleiberit Euroleim-300" - universal application, based on polyvinyl acetate dispersion; used for gluing cases, laminated boards, MDF boards, etc.: adhesives "Tempo-305", "Tempo-332", "Tempo-338", "Tempo-347" - all of them based on polyvinyl acetate dispersion; used for gluing layers of laminated polymer plastic, laminates. The same company (Kleiberit) produces high-quality hot melt adhesives of several grades: SK-774.4; SK-774.8; SK-777; SK-779.6; SK-779.7; SK-782.1 (all in the form of granules). They soften at temperatures from +105 °C to +115 °C; applied at temperatures from +200 to +240 °C; are used in furniture production for gluing veneer, decorative elements, film materials, when facing profile parts and edges. 4. Rubber adhesives In construction, shoe and furniture production for many years at the end of the XNUMXth and the beginning of the XNUMXst century. widely used rubber adhesives, manufactured on the basis of natural or synthetic latexes and solutions based on rubber compounds. The most common are latex adhesives based on a copolymer of divinyl with methyl methacryl and polychloroprene - grades KL-1, KL-2, KL-3, which are used when lining panel parts with wood veneer, films based on paper and polyvinyl chloride. For many years, the well-known adhesive "Bustilat" has been used in construction, containing up to 41 mass parts of SKS-65 GP latex; it is applied to a sticker of linoleums, textile materials and many other materials. Latex adhesives good adhesion of various materials with porous surfaces. In shoe and furniture production, as well as in everyday life, rubber adhesives are used in a large assortment and quantity, obtained on the basis of solutions of natural and synthetic rubbers in organic solvents. Various modifiers, antioxidants, plasticizers, and hardeners are added to these adhesives. Solvents are acetone, ethyl acetate, toluene, methyl etiketone, etc. These adhesives are used for gluing polyurethane foam, sponge rubber to each other, as well as for sticking to wood, cardboard, wood fiber and other materials. In shoe production, when repairing shoes in workshops, ateliers and at home, as well as in furniture production, nairite adhesives are constantly and in large quantities used. The most widely used (for many years) nairite adhesives of grades: 88N, 88NP 88NP-35 and NT, used in the cold gluing method. The most common and most commonly used adhesives are 88N and 88NP, designed for gluing various shoe materials (natural and artificial leather, fabrics, plastics), as well as for gluing rubber, foam plastics, fabrics to each other and for gluing them to metal, concrete, wood . Adhesives 88NP-43 and 88NP-130 are used for gluing rubber and foam rubber to metal, facing and flooring materials to wood and rigid foam materials. Russian industry produces a whole range of chlorine-irite adhesives based on chlorinated chloroprene rubber and nairite. Of these, the glue brand "KS-1", consisting of nairite grade A, chlorinated nairite, magnesium oxide, zinc oxide, diphenylguandine, has become widespread. These adhesives are used in the same cases as the adhesives of group 88H. For a long time (more than 30 years), adhesive rubber mastics KN-2 and KN-3 have been used in construction, which are a viscous paste-like homogeneous mass containing chloroprene rubber, indencoumarone resin, fillers and solvents. Mastic KN-2 is intended for gluing rubber linoleum and rubber tiles and plates. Mastic KN-3 is used for gluing coatings with a porous layer, nitrolinoleum, profile moldings. Adhesive rubber mastics are flammable and explosive, as well as toxic materials. 5. Protein adhesives In the second half of the XX century. widely used in construction protein adhesives - mezdrovy, bone and casein. They were also used in the furniture industry. In construction, these adhesives were used to prepare various paint compositions, in furniture production - for gluing wood. Casein is a protein substance that is secreted in the form of curd mass during the souring of milk. To get glue, some alkali is added to the water in which the casein is (in lumps): soda, potash or ammonia. Under the action of alkali, casein dissolves and after an hour it turns into glue, which, in combination with lime, gives an indelible paint (if a coloring pigment is also added). Industry releases casein glue in the form of a powder with the addition of the necessary components - two brands: "Extra" (B-107) and "Ordinary" (OB). Casein glues give sufficiently strong and elastic connections - the strength of wood bonding, not less than: for "Extra" glue - 10,6 MPa, for "Ordinary" (OB) glue - 7,5 MPa. Casein adhesives are used in furniture production for gluing thick sheet materials in the manufacture of furniture panels, for gluing wood, decorative laminated paper. Proteins include collagen adhesives - mezdrovy and bone, in which the adhesive is a protein - collagen, contained in the connective tissues and bones of animal organisms. In cold water, collagen swells, and when heated, it passes into a new substance - glutin, which has the properties of glue. Skin glue subdivided into solid and gallerty. Solid hide glue is produced by tile, flake, chip, crushed and granular. Hide glue is obtained by boiling protein waste from tanneries and leather raw materials factories with water, followed by drying. (Mezdra is the subcutaneous layer of an animal's skin.) Mezdra glue is used in construction and furniture production. bone glue (collagen) is produced from defatted and polished animal bones. This glue is produced in several types: galerta (glue jelly), tile, crushed granular and flake. All types of bone glue are divided into grades: the highest, 1, 2 and 3rd. Boiling the bones first forms a thin broth, which is then evaporated to a deep yellow or brown jelly. Such glue is called galley. Glue solutions based on bone glue can rot after a while, so they are injected with one of the antiseptics, such as phenol or formalin. In painting work, bone glue is used for the preparation of paint compositions, primers, putties, lubricating pastes. Currently, protein adhesives are produced in small quantities and have limited use, as they have been replaced by synthetic adhesives that have 100% biological stability, high moisture and heat resistance, and low drying shrinkage. Protein adhesives do not possess these qualities. 6. Adhesive films and tapes In the woodworking industry and furniture production, over the past two decades, adhesive films and tapes have been widely used, which have a layer sticky adhesive that retains stickiness for a long time. When applied to the surface of any material, these tapes and films adhere to it when pressed. Adhesive films and tapes are based on the following materials: Sulphite paper weighing 20 g/m 2, polyethylene, cellophane, fabric, plasticized polyvinyl chloride, etc. Various elastomers and polymers with various additives are used to apply an adhesive layer to the base. The following are most widely used in woodworking and in the manufacture of furniture from wood. adhesive films and tapes: 1) bakelite film (GOST 2707), used for gluing aviation, decorative and birch plywood, furniture blanks; glued at a temperature of + 150-155 ° C and a pressure of 2-2,5 MPa; 2) adhesive tape (GOST 18251), designed for gluing veneer strips into full-length sheets; before use, the coating of the tape is moistened; 3) paper adhesive tape (TU 13-7309005-669-88). It is used for applying to the edge material during its manufacture and for protecting the edge of the shield during its finishing; 4) adhesive tape LPLO-M (TU OP 13-64-37-83). Designed for gluing the ends of paper rolls during reloading, breakage of fasteners to the winding sleeves during the impregnation process; 5) adhesive tapes LT-38, LT-50 on a polymer basis. They are used to protect the edging material from drips of varnishes and paints when finishing furniture panels, as well as to protect the edges from mechanical impacts during transportation. These tapes (adhesive) are a polymer base film with a thickness of 35-50 microns, on which a thin adhesive layer is applied. LECTURE No. 16. Finishing materials 1. Appointment of finishing materials. Materials for surface preparation for finishing The purpose of finishing materials is to protect buildings, various structures and furniture from environmental influences or to improve the appearance, as well as to increase the service life. In construction, for exterior decoration of buildings and structures (in the exterior), plastering, facing with marble, granite, ceramics, decorative bricks, modeling, decorative painting. In the interior decoration (interior), the same materials are used as in the exterior, as well as wallpaper, linoleum, joinery, synthetic materials (plastics). In the furniture industry, for protective and decorative coatings, a wide variety of finishing materials are used, which are divided according to their purpose into the main groups: for preparing wood before applying a paint and varnish coating; to create a paint layer; auxiliary. Primers - these are compositions that include pigments, fillers and binders, which differ from painting compositions in a lower content of pigments. The purpose of primers is to equalize the "pulling" ability of the surface, to make its porosity the same. For furniture production, primers are used in the form of solutions of resins, nitrocellulose and plasticizers in a mixture of solvents. The following brands of primers are used in construction: glyptal GF-032, GF-020 and others; perchlorovinyl XB-050, XB-785, polyvinyl acetate VL-02, VL-02A, VL-023A. Primers for furniture production are used in the following grades: NK, BNK, PE-0155, etc. Putties - These are thick viscous mixtures in the form of pastes, consisting of pigments and fillers in a binder. They serve to fill irregularities and correct defects in the painted surface. The following putties are used in construction: MS-006 - alkyd-styrene; perchlorovinyl - XB-004, XB-005, etc.; polyvinyl acetate; epoxy EP-0010, etc. A wide variety of putties are used in furniture production: polyester putties - P7-0025, P7-0059; epoxy - EP-0010; perchlorovinyl - XB-004, XB-005; varnish putties based on oil and alkyd varnishes - No. 175, LSh-1, LSh-2; adhesive putties, which are prepared at the point of consumption. Fillers and compositions of fillers applied under transparent coatings, while they help to reduce the consumption of paints and varnishes and reduce subsidence of the coating. The following fillers are used in construction and furniture production: KF-1, similar - KF-2, KF-3, KF-4; fillers TMB-1, TMB-3, TMB-4 are one-component pastes that do not contain vegetable oils. Dyes in construction and furniture production are used in a variety of ways: dyes (synthetic, acid and natural); porenbeytsy - liquid paints and varnishes for dyeing wood; mordants (chemicals - iron, copper sulfate, etc.). Pigments - finely divided powders of various colors - are used in a mixture with a solution of a film-forming composition that fixes the pigment powder on the surface. 2. Lacquers and polishes for clear finishes Various varnishes and varnishes are widely used in furniture production and construction. Laki are solutions of natural or synthetic film-forming substances in organic solvents or water, which after drying form a transparent solid homogeneous film with good adhesion to the material being trimmed. Lacquers are divided into alcohol, nitrocellulose, polyester, urea-formaldehyde, as well as varnishes that form films due to the joint process of evaporation of solvents and chemical reactions; oil varnishes (have limited use - due to the duration of drying and the lack of oils). Oil varnishes - these are solutions of resins - rosin, copal, glyphthalic in oils - linseed, hemp, tung and their solvents - turpentine, xylene, white spirit, etc. with the addition of desiccants (to speed up the drying of the varnish coating). Widely used in various sectors of the economy, including the manufacture of furniture and construction, several types of urea-alkyd varnishes: MCH-52, MCH-270, ML-2111 - for furniture, skis, musical instruments; ML-2111 PM - for finishing film materials. At present, they are widely used in the furniture industry and construction. nitrocellulose high quality varnishes, manufactured by the German company Herberts: cellonite D-1009, D-1013. Nitro-lacquers have unlimited viability, they are quite technologically advanced. Domestic enterprises produce cold-applied nitro-varnishes of the NTs-218, NTs-221, NTs-222, NTs-224 brands, which form transparent, shiny coatings on the surface, with the exception of NTs-243 varnish, which forms transparent matte silky coatings. In recent years, polyurethane varnishes "Contracid D-3010", produced by the German company Herberts, have been used for high-quality finishes, colorless, used to cover parquet and plank floors, finish bathroom products, kitchen and office furniture. This varnish forms coatings of highly wear-resistant, light -, chemical and moisture resistant. PF-283 (a solution of alkyd resins) is most commonly used for interior coatings on metal, light-colored wood, furniture and light-colored oil paints, for cars and railway cars. Widely used in furniture industry varnishes different types: alcohol and nitro polishes, which penetrate deeper than varnishes into the wood and form very thin films with gloss and elasticity, while they allow you to clearly reveal the grain of the wood. Polishes are low-concentrated solutions of polishing bodies. Alcohol polishes - a solution of shellac resin in ethyl alcohol, the most common shellac polish - 10-20% alcohol solution of shellac (produced under the number - 13, 14, 5 and 16), used for polishing shellac, nitrocellulose and oil films. Nitropolishs are used for polishing nitro-lacquer coatings after leveling and grinding. Most often, domestically produced nitropolish NTs-314 is used. 3. Paints and enamels for opaque finishes Various paints and enamels are widely used for opaque finishing of various surfaces in construction, furniture production and in almost all sectors of the economy as a whole. Paint are made in the form of a mixture of finely divided pigments and fillers with a solution of film-forming substances. Depending on the purpose, type of film-forming substance, pigment and filler, ready-to-use oil, alkyd, silicate, organosilicate, water-based, perchlorovinyl, cement and other paints are produced in a variety of colors. Paints, depending on the purpose, are produced for outdoor and indoor use. For exterior work - painting brick, concrete, plastered and other porous external surfaces, primed metal surfaces, as well as old coatings - water-based paints based on aqueous dispersions of synthetic polymers of the following grades are used: E-AK-111, E-VA-17, E -VS-114, E-KCh-112. The Moscow plant "Svyatozar" (paint and varnish), since 1990, has been producing high-quality paints: facade "Svyatozar-15" - acrylic, matte, white (tinted in pastel colors). In recent years, many imported paints have been supplied to Russia from Finland (TIK-KURILA), Germany (JOBI, KIMEG), and Great Britain (HAMMERITE). At present, the following domestic paints are widely used: oil paints MA-15 (all colors), water-based paints VDAK-2180, facade paints - KhV-161, VDAK-1180, KO-815, KO-868, AK-124, paints for road marking - AK-591; as well as PF-115, VDVA-201, NTs-132, ML-12, VDKCH-224, VD-205, VA-17 - for outdoor and indoor work. Enamel are suspensions of pigments in varnishes with the addition of plasticizers and desiccants, they are used in the same way as paints for exterior and interior work on metal, wood and plaster. Enamels differ from paints in their increased content of film-forming substances, which provides coatings with higher decorative qualities. Enamels are produced by the industry in finished form of the following grades: oil, oil-glyphthalic - GF-1426, GF-230, etc.; pen-taphthalic - PF-223, PF-115, PF-266, etc.; nitrocellulose (quick-drying, widely used in furniture production) - NTs-132, NTs-25, NTs-11A, NTs-257, NTs-257, NTs-251, NTs-273, etc .; polyester enamels - PE-225, PE-276, V-PE-P79, etc. Perchlorovinyl give coatings that are resistant to the action of chemical reagents and atmospheric phenomena (including acid rain), - XB-124. Oil-glyphthalic and oil enamels are used for interior decoration of premises, offices, metal and wood products used indoors. Pentaphthalic enamels are suspensions of pigments in pentaphthalic varnish with the addition of a desiccant and solvents, designed for painting metal and wooden surfaces that are not exposed to atmospheric influences, are widely used in household construction. 4. Drying oils Drying oil is an oily liquid, which, after being applied to the surface, dries, forming a strong elastic waterproof film. Drying oil is produced by processing vegetable drying or semi-drying oils, fats and organic products that do not contain varnish resins. Drying oils are divided into four types: natural, compacted, combined, synthetic. Natural drying oils obtained by processing (cooking) vegetable oils at a temperature of + 200-300 ° C, while adding a desiccant to the oil, for example, oxides, peroxides and salts of lead, cobalt, manganese. Cooking oil and adding a desiccant accelerate the drying (hardening) of the films after applying the paint to the surface. Compacted or semi-natural drying oils are the product of compaction of vegetable oils by oxidation, polymerization or oxypolymerization, which is then diluted with a solvent. In the production of such drying oils, significant oil savings are achieved (up to 45%). Combined drying oil obtained on the basis of drying and semi-drying oils, which are subjected to polymerization and dehydration; a mixture of polymerized and dehydrated oils is also used, mainly for the preparation of thickly grated paints. Synthetic drying oils are made from synthetic resins (polymers) or various oils by thermal and chemical treatment. Such drying oils, after being applied to the surface, harden, forming a thin film. The most important type of synthetic drying oils are alkyd drying oils (glyphthalic, pentaphthalic). Synthetic drying oils are used for the preparation of thickly grated and ready-to-use oil paints. These drying oils contain 50% alkyd resin and 50% drying oil. Natural linen and hemp drying oils produced from linseed or hemp oil with the addition of drying accelerators - manganese, lead and cobalt driers. Natural linen and hemp drying oils are used for the manufacture and dilution of thickly grated paints, as well as an independent material for painting. Semi-natural drying oil oksol is a solution of oxidized vegetable oil and desiccants in white spirit. Depending on the raw materials used, it is produced in two grades: "B" - from linseed and hemp oils; "PV" - from sunflower, soybean, safflower, corn, grape oils. Oil paints used for exterior and interior work are made from drying oil of grade "V", and grades "PV" are used for paints used only for interior work, with the exception of floors. Drying oil polymerized - substitute for natural drying oil; obtained by compacting heated linseed oil and then adding solvent and desiccant. It is used to dilute thickly grated paints for exterior and interior painting on metal, wood and plaster in buildings and structures of the first and second classes. Glyphthalic drying oil is also used in finishing works, which is produced by the interaction of vegetable oils, glycerin and phthalic anhydride in the presence of a desiccant. This drying oil is diluted with thickly grated paints intended for interior and exterior painting on metal and wood. LECTURE No. 17. Floors 1. Types of floors The device and type of floors in the construction of various buildings and structures are determined by building codes and regulations (SNiP). Depending on the purpose of buildings and structures, the floors inside them - in the premises can be very diverse: wooden, polymeric, ceramic, glass and slag glass, asphalt, concrete with a mosaic coating of Breccia-type slabs. Breccia is a 400 x 400 mm or 500 x 500 mm slab made of fragments of marble, granite, ceramics on an epoxy adhesive basis. In the mechanical workshops of enterprises, where auto or electric cars carrying various cargoes operate, asphalt floors are covered from above with metal perforated or corrugated slabs of 500? 500 mm or less. In the production premises of various enterprises, mosaic floors are also installed using marble chips based on special cement mortars. Such floors, after drying the solution in the crumb component, are sanded using special grinders. In addition, in public buildings and auxiliary workshops of various enterprises, slabs of glass-silica, glass crystallite and slag glass-ceramic are used for flooring. Such floors are characterized by high decorativeness, durability, alkali and acid resistance. In the shops of various chemical industries, multi-layer floors with acid- and alkali-resistant coatings are being equipped. The bottom layer is a coating of polyisobutylene or rubber plates with special properties (resistant to aggressive environments), and the top layer is acid-resistant ceramic tiles laid using special solutions. Decorative ceramic tiles of various types are used everywhere in the construction of floors in sanitary facilities, baths, laundries, lobbies and halls of various buildings. The use of ceramic tiles for flooring ensures long-term operation, reduces the cost of repairs (only parts of the floors damaged during operation are changed). Floors made of ceramic tiles also have such qualities as: water resistance, acid and alkali resistance, good abrasion resistance, easy to clean, disinfectant solutions can be used, various patterns (ornaments) are formed. In recent years, various polymer coatings have been widely used in the arrangement of floors: linoleum, polymer tiles, synthetic carpets, self-leveling seamless polymer coatings. Polymer floors in the total volume of floors make up 40%, they are installed in public buildings, auxiliary premises of various industries, sometimes in offices, offices, apartments. According to existing building codes, wooden floors are laid in schools, children's and medical institutions, and in residential buildings. These floors are made using floorboards, floorboards and parquet products. In recent years, parquet floors have become widely used in private construction, in offices in the form of piece parquet boards, parquet boards, parquet panels; with mosaic and artistic and decorative design. 2. Materials and products for wooden floors For a long time, in the construction of residential buildings, various buildings and structures, plank floors were arranged, for which mainly products made from oak, beech, maple, ash, larch, spruce, pine, etc. were used. Linden and poplar wood is not allowed. Before the advent of woodworking machines, boards and blocks of wood were simply fitted together. Then, after the invention of machine tools, materials for floors began to be processed by milling. For a snug fit of the floorboards - the front sides - their lower part is already made by 1 mm, and on one edge there is a groove, on the other - a comb.  Rice. 11. Boards for flooring: a - DP-27; b - DP-35; c - bar BP-27 Floorboards are made of three types - the first, second and third. Floorboards of the third type usually have a thickness of 37? 40 mm and are used when laying wooden floors in industrial buildings, sports halls and other premises with increased load on the floors. In residential buildings, floor boards with a thickness of 25? 35 mm, which are laid on transverse bars (wooden) - logs of size 40? 40mm or 50?? 50 mm. The wood from which the floorboards and bars are made is preliminarily subjected to antiseptic and impregnation with fire-fighting compounds (flame retardants). In addition, to protect the floorboards and bars (logs) from insects, treatment is used by fumigating with toxic gases in special heating chambers to a temperature of + 100 ° C or a highly effective preparation "Ermit" is used (provides protection against biological influences and fire resistance of wood for 20 years). The following requirements are imposed on floorboards and bars: humidity 12? 3%, the roughness of the front surfaces for a transparent finish - not less than 80 microns, for an opaque finish - not less than 200 microns, and for non-facial surfaces - not less than 50 microns. Accounting for wooden materials for floors is carried out in cubic meters, while their width is measured without taking into account the height of the ridge. 3. Materials and products for parquet floors In recent years, the use of piece, mosaic parquet, parquet boards, parquet panels in private construction (cottages, mansions, summer cottages) and on orders for repairing floors in buildings in operation has increased. In serial construction, parquet is rarely used because of the high cost and laboriousness of the work. Parquet floors are usually laid in residential premises, public buildings, auxiliary premises of industrial enterprises. Block parquet is a plank of oak and tropical wood (grade A), as well as beech, elm, ash, maple, chestnut, hornbeam, larch (grade B). Brand A corresponds to the highest category, and brand B - to the first. Mosaic parquet It is made in the form of shields, subdivided into two types (according to the method of attaching the slats to the base): 1) P1 - planks are glued with the front side on the paper, which is removed after parquet flooring; 2) P2 - the strips are glued with the reverse side to an elastic (heat and sound insulating) bioresistant material. which remains in the floor structure after parquet flooring. This type of parquet is also subdivided into grades A and B, depending on the quality category, wood species and plank processing. The thickness of planks of mosaic parquet made from hardwood is 8 mm, from softwood - 10 mm. Length of parquet planks - from 100 mm to 230 mm, width - from 20 to 30 mm. Parquet floors sometimes they are made of parquet boards, which, depending on the design of the base, are divided into three types: 1) PD1 - with a single-layer base of laths typed in squares or rectangles located mutually perpendicular; 2) PD2 - with a single-layer base made of laths assembled in the direction of the longitudinal axis of the parquet board; 3) PD3 - with a two-layer base of two layers of laths or laths and veneer glued together, laid in a mutually perpendicular direction. Parquet boards consist of a base in the form of laths and a covering of parquet planks or veneer. The dimensions of the parquet planks on the board (coating) are as follows: length - from 150 to 207 mm, width - from 20 to 50 mm; thickness - 6 mm. For the device of parquet floors, parquet boards are often used, which have dimensions: from 400? 400 mm to 800? 800 mm, thickness from 22 to 40 mm. These boards consist of a base on which parquet planks are glued according to a specific pattern. On the edges of parquet boards, grooves are made for their connection with dowels. Parquet planks on such panels have the following dimensions: length - from 100 to 400 mm, width - from 20 to 50 mm, thickness - 6 mm. In recent years, artistic parquet has been used for finishing floors in VIP salons, offices, cottages, mansions, villas - as a kind of panel parquet. Artistic parquet is made in two main ways: "marquetry" - when the pattern is collected from individual planks, different in color and texture, tightly fitted one to the other; "intarsia" (inlay) - individual fragments of wood of other species with different textures and colors are inserted into the main background of the front layer of wood. 4. Polymeric materials and products for floors For several decades, in the arrangement of floors in public buildings, auxiliary premises of industrial enterprises, polymeric materials and products have been widely used - in the form rolled (linoleum, synthetic floor coverings of all kinds), tiles, sheets, as well as mastics, polymer-cement and polymer-concrete compositions. The most widely used for the arrangement of floors is polyvinyl chloride linoleum. Linoleum, depending on the structure, is produced by the industry of three types: "MP" - multilayer with a front layer of a transparent polyvinyl chloride film with a printed pattern; "M" - multi-layer one-color or marble; "O" - single-layer one-color or marble; in the form of rolls 12 m long, 1200-1400 mm wide and 1,5 and 1,8 mm thick. For flooring, polyvinyl chloride linoleum is often used on a heat and sound insulating basis. Such linoleum is used for arranging floors in rooms where there is no exposure to abrasive materials (such as sand), fats, oils, water and solutions of aggressive chemical materials. Polyvinyl chloride linoleum on the base has two layers: the bottom is a non-woven needle-punched material that serves as a heat and sound insulating base, the top is covered with a transparent front polyvinyl chloride film with different patterns or one color; total thickness of 2 layers - 3,6 mm, width - 1350 mm, roll length - 12 m. When installing floors in utility rooms of industrial enterprises, multilayer rubber linoleum (relin) is often used, made from rubber compounds based on synthetic rubbers - one-color or multi-color, with a pattern, in rolls 12 m long, 1000 mm wide and more, 3 mm thick. This type of linoleum does not shrink during operation, is hygienic, has increased sound absorption. For more than half a century in construction, when arranging floors in industrial and public buildings (in utility rooms), PVC tiles, one or multi-color with a smooth or embossed front surface, size 300? 300 mm thick 1,5 and 2,5 mm - square or trapezoidal. In recent years, to cover various floors (plank, cement) began to be used synthetic carpet materials - lint-free and with pile. The most widely used for arranging floors in offices, cottages, mansions is synthetic carpet, as a pile, the bottom layer of which is a PVC backing, and the top layer is a loop pile made of synthetic fibers or a mixture of synthetic and chemical fibers. This coating is produced in rolls 12 m long, 1,5-2 m wide and 3-5 mm thick. Imported similar carpets have a width of 2, 3, 4 and 5 m. In the construction of industrial enterprises for many years, various pasty mastic polymeric materials for seamless flooring (concrete or reinforced concrete bases). LECTURE No. 18. Building materials 1. Natural stone materials Natural stone materials have been used in construction since time immemorial. The main and widely used natural stone materials are sand (mountain and river), gravel, chalk, kaolin, crushed stone, which belong to rough stone materials In addition to the listed materials, processed stone materials are used in construction: sawn piece stones and blocks for walls, stones, slabs and profile products with variously processed surfaces for external and internal cladding of buildings and structures. According to existing building codes and regulations, natural stone materials are classified according to the following criteria: bulk density - heavy - with a bulk density of more than 1800 kg / m 3 and light - less than 1800 kg/m 3; by compressive strength - for grades: 4, 7, 10, 15, 25, 35, 50, 75, 100, 125, 150, 200, 300, 400, 500, 600, 800 and 1000 - respectively from 0,4 up to 100 MPa. The most common and used stone material in construction is mountain and river sand. No less widely used in construction are such stone materials as chalk and kaolin. Chalk is a sedimentary rock whose chemical composition is pure calcium carbonate CaCO 3. In the construction and paint industry, kaolin is also widely used - a product of the destruction of rocks containing feldspars. Kaolin is a white clay, chemically it is a hydrous aluminum silicate; added to colors containing chalk to improve their painting and technical qualities. For road construction and in the manufacture of concrete and reinforced concrete products, dolomite or granite gravel is constantly and in large volumes used, which is a product of the destruction of rocks, in the form of small or relatively large stones (polished with water) ranging in size from 5 to 75,0 mm, medium density over 2 g/cm 3. Gravel on frost resistance is divided into grades: Мpz 15, 25, 50, 100, 150, 200, 300. Crushed stone is constantly and in large quantities used as a large aggregate for concrete of monolithic, prefabricated concrete and reinforced concrete products, as well as in road construction - in the form of irregularly shaped stone pieces ranging in size from 15 mm to 150 mm. Crushed stone is natural (grass) and crushed. Crushed crushed stone is obtained by crushing large pieces of rocks in crushed stone plants. According to frost resistance, crushed stone is divided into the following grades: Mpz 15, 25, 50, 100, 150, 200, 300. Rubble stone is used similarly to crushed stone - in construction for foundations, as a filler for rubble concrete in the construction of concrete and reinforced concrete massive structures, in the laying and repair of roads. The sizes of pieces of rubble stone - from 150 to 500 mm. According to frost resistance, rubble stone is divided into grades: Mpz 15, 25, 50, 100, 150, 200, 300. In construction, various stone wall materials are widely and in large volumes - bricks, stones, small blocks and slabs, which are divided into ordinary ones, intended for laying external and internal walls, and front ones, for wall cladding. 2. Concrete The use of various types of concrete is an important part of the construction of various industrial and civil facilities. Concrete is an artificial stone material obtained by shaping and hardening a concrete mixture consisting of a binder, water, aggregates and special additives in a certain proportion. According to the type of aggregate, concretes are: on dense aggregates, on special aggregates, on organic aggregates. Depending on the bulk density, concretes are subdivided: especially heavy - with a bulk density of more than 2500 kg / m 3; heavy - 2000-2500 kg/m 3; lightweight - 1800-2200 kg/m 3; light - 500-1800 kg/m 3. Lightweight concretes are made of the following types: on porous and artificial aggregates; cellular concrete coarse-porous concrete; especially light - with a bulk density of less than 500 kg/m 3. Concrete is prepared directly at construction sites using concrete mixing plants of various sizes. For the manufacture of various concrete and reinforced concrete structures, heavy (structural) concretes are used, prepared on a cement binder, dense large and small aggregates. Lightweight concretes are prepared using a cement binder and porous coarse aggregate or dense fine aggregate, and are used in industrial, agricultural and other types of construction. For lightweight concrete, the following classes and grades are established: strength classes for structural concrete - B2,5; B3,5; B5…B40; strength classes for heat-insulating concrete - B0,35; B0,75; IN 1. Light concretes include arbolite, made on a cement binder, organic aggregates and chemical additives Arbolite and products made from it are intended for use in buildings for various purposes with a relative humidity of indoor air of not more than 60% and in the absence of aggressive media (liquid and gaseous). In construction, cellular concretes are often used, which, depending on the purpose, are divided into heat-insulating, structural-heat-insulating structural and special, in addition, they are divided according to the type of pore formation into aerated concrete and foam concrete. According to the conditions of hardening, cellular concretes can be autoclaved and non-autoclaved. For cellular concrete, the following classes and grades are established: compressive strength classes - B0,35; B0,75; IN 1; B1,5; … IN 20; grades for medium density - D300, D400, D500 ... D1200. Silicate concrete grades for medium density - D1000, D1100, D1200 ... D2400. Concrete plants also produce heat-resistant concretes intended for products, structures and facilities operating at temperatures above +200 °C. For the manufacture of products and structures operating in various conditions, exposure to aggressive environments, chemically resistant concretes are produced based on furan, furan-epoxy, urea, acrylic synthetic resins (polymer concrete) and liquid sodium or potassium glass with a polymer additive (polymer silicate concrete). 3. Mortars In the construction of buildings and structures using bricks, wall blocks and panels, various mortars are used. When brickwork is performed, installation of wall blocks and panels, cement mortars are used, which have compositions from 1: 1 to 1: 6, i.e., from 1 to 6 parts of sand are taken for one volumetric part of cement (mortars 1 are most often used: 1 and 1:2). Cement mortars in a ratio of 1: 3 or 1: 4 are used for plastering the lower parts of foundations in a humid environment, plinths and exterior walls of buildings. In addition to cement mortars, other types of mortars are also used: lime, gypsum и mixed. All solutions are subdivided according to the average density in the dry state - into heavy ones, with an average density of 1500 kg / m3 and more, and light, with an average density of less than 1500 kg / m 3; marks are set according to the limit: 4, 10, 25, 50, 75, 100, 150, 200. The brand of mortar and the ratio in the compositions depends on the brand of cement. For example, the brand of cement mortar 50, with the use of cement M-400, the composition will be 1: 6 (for one part of cement - 6 parts of sand). Cement-lime mortars (mixed) are used for plastering exterior walls, wetted parts of the building. Compositions of cement-lime mortars (cement: lime paste: sand) in volume parts: 1: 1: 1; 1:2:8; 1:2:11 and 1:3:15; these proportions depend on the brand of cement. Lime-gypsum mortars are intended mainly for plastering wooden surfaces of non-moistened rooms, as well as stone, fiberboard surfaces. Lime mortars are used for plastering dry rooms, structures made of stone, brick, wood and adobe. The amount of sand added to the solution depends on the "fatness" of the clay. To improve the strength and quality of work during masonry and plastering, organic plasticizers - microfoam formers (soap naft, soapy lye, soap stock waste, etc.) are introduced into the composition of mortars. For finishing the facades of buildings and interiors of internal premises, as well as for the factory finishing of the front surfaces of wall panels and large blocks, decorative mortars are used: terrasite, cement-sand, lime-sand. To obtain the desired design, coloring additives are introduced into these solutions for decorative plaster - pigments (light-resistant, alkali-resistant and acid-resistant - natural and artificial). To fill the channels of prestressed reinforced concrete structures, so-called injection mortars are used - cement-sand and cement. For laying industrial furnaces and other thermal units made of aluminosilicate bricks, a special fireclay-cement mortar is used, which is heat resistant. Fireclay-bauxite mortar is also used for similar work (when laying elements of furnaces operating at temperatures from +1300 to +1350 ° C). In the manufacture of these heat-resistant solutions, Portland cement and plasticized Portland cement are used as a binder in fireclay-cement mortars, and sodium liquid glass with a modulus of 2,5-3 is used in fireclay-bauxite mortar. 4. Inorganic aggregates for concrete Non-metallic building materials, crushed stone, slag and sand from waste products of various industries, as well as porous natural and artificial materials are used as inorganic aggregates for concrete. Crushed stone and sand from industrial waste (mining and processing) are classified as dense materials. porous natural materials are tuff and pumice of volcanic origin. coarse aggregates are crushed stone and gravel, fine - sand. Slag crushed stone is used as a large dense aggregate in the manufacture of heavy concrete of prefabricated and monolithic concrete and reinforced concrete structures, parts of buildings and structures. By strength, crushed stone from dense metallurgical slags is divided into the following grades: DR 15, DR 25, DR 35, DR 45. In the manufacture of lightweight concrete (heat-insulating and structural), gravel and expanded clay sand are used as aggregates. These materials are porous artificial aggregates. Expanded clay sand is obtained by crushing expanded clay gravel. Depending on the density, the gravel of each fraction is divided into grades: 250, 300, 350, 400, 450, 500 and 600. Expanded clay sand, depending on the density and fraction, has grades from 500 to 900. In the manufacture of structural and structural-heat-insulating lightweight concrete, crushed stone (gravel) and thermolite sand are used as fillers. For the manufacture of structural lightweight concrete, crushed stone and agloporite sand are widely used as aggregates, which are obtained by crushing cakes formed as a result of agglomeration of a granular charge composed of natural mineral raw materials and industrial waste. In the manufacture of heat-insulating and structural lightweight concrete, in addition to the above porous artificial aggregates, shungizite gravel and sand are used. Such gravel is obtained by firing shungite-containing rocks, and sand is obtained by crushing this gravel. Shungizite gravel of each fraction, depending on the bulk density, is divided into grades 200, 250, 550, and sand from the above gravel - into grades 500-900. In construction, sand and crushed stone, expanded perlite, obtained by grinding and heat treatment of volcanic water-containing rocks, have been widely used for a long time. These materials are used in the manufacture of lightweight concrete, and perlite sand is also used for heat-insulating backfills, plaster mortars, heat and sound insulating materials, and products. Grades of expanded perlite sand by bulk density - from 75 to 500, and crushed stone - from 200 to 500. For more than fifty years, such a wonderful material as expanded vermiculite has been used in construction as a heat-insulating backfill at a temperature of insulated surfaces from -260 ° C to +100 ° C. The raw materials for obtaining expanded vermiculite by firing are natural hydrated micas. Vermiculite grades by bulk density - 100, 150, 200. 5. Products based on mineral binders For many years (in the XX-XXI centuries), various products based on mineral binders have been widely used in construction. The most common in the production of construction works are gypsum and gypsum concrete products. Gypsum boards are often used for partition walls in buildings with dry and normal room conditions. Widely used in the XNUMXth century. And now plasterboard sheets are used for finishing and arranging walls and partitions in buildings and rooms with dry and normal humidity conditions, as well as for the manufacture of decorative and sound-absorbing products. For the installation of load-bearing partitions in buildings for various purposes, gypsum concrete panels are used, made of concrete on a gypsum or gypsum-containing binder. Everywhere in construction, various asbestos-cement products are used: flat and profiled sheets, slabs and panels - for walls and coatings, pipes and fittings. Products made of asbestos cement have many valuable properties: frost resistance, water resistance, high thermal conductivity, are easily polished and can be machined, do not rot, and are fire resistant. For a long time, cement-sand tiles, made from a mixture of Portland cement, sand and clay, have been and are being used in construction (for arranging roofs). Such tiles have the following dimensions: length 390 mm, width 240 mm and thickness 8-10 mm, the depth of the grooves is about 5 mm, and the height of the spikes for suspension is at least 10 mm. For attachment to the roof lathing in the tile, one through hole is made in the overlapped part during manufacture. In cities, for the installation of prefabricated pavements of sidewalks, garden and park and pedestrian paths, landing sites on public transport lines, concrete sidewalk slabs are widely and widely used, made from heavy concrete with various additives that ensure the long-term operation of such products. Concrete paving slabs are made in the form of a square, rectangle, regular hexagon or curvilinear closed figures. On the basis of mineral binders, various architectural and construction products are manufactured in the form of decorative facing slabs with mosaic, ornamental surfaces, intended for external and internal cladding of elements of buildings and structures. Concrete facade slabs are also manufactured, used for facing walls and plinths of stone buildings and structures. In addition to the above products in construction (mainly low-rise), wall concrete stones are used for load-bearing and enclosing structures of residential, public, industrial and agricultural buildings. These stones are made in the form of full-bodied and hollow rectangular parallelepipeds, they are ordinary and facial. The latter are made with painted and unpainted front surfaces. Concrete wall stones are produced by concrete plants of the following types: "SKTs" - on a cement binder; "SKI" - on lime; "SKSH" - on slag; "SKT" - on a gypsum binder. 6. Prefabricated concrete and reinforced concrete products The use of prefabricated concrete and reinforced concrete products forms the basis of capital construction in many sectors of the country's economy. In housing and civil construction, a wide range of prefabricated concrete and reinforced concrete products is used: reinforced concrete monolithic foundations, wall blocks, reinforced concrete floor panels, flights of stairs and landings, wall and partition panels, window sills, crossbars, balconies, blocks of sanitary facilities, parapets and other products . Reinforced concrete is the main building material, which combines concrete (various types - light, heavy, etc.) and steel reinforcement (made of special steel grades 35GS-AP, A - III, A - IV classes), located in a stretched zone of the structure and perceiving tensile stresses Compressive stresses are transmitted to concrete in such a product. Reinforced concrete structures are monolithic, concreted at the construction site (poured monolithic reinforced concrete foundations, as well as load-bearing walls of buildings - using special formwork - according to a new technology for the construction of residential buildings), and prefabricated, assembled at the construction site from individual elements (wall panels or blocks in large-panel housing construction). For industrial construction, a large range of prefabricated concrete and reinforced concrete products is also used: foundation blocks (FBS-4, FBS-5, etc.), pillows, beams, crossbars, prefabricated concrete and reinforced concrete piles, trusses, arches, crane beams, stair marches and platforms, etc. In transport construction, reinforced concrete sleepers (instead of wooden ones), elements of passenger and cargo platforms, reinforced concrete culverts and pipes, as well as prefabricated concrete and reinforced concrete superstructures of bridges are widely used. A wide variety of prefabricated concrete and reinforced concrete products is used in agricultural construction: foundation blocks (FBS1, FBS-2, FBS-3, etc.), foundation cushions, cast-in-situ reinforced concrete foundations, trusses, pile-columns, beams, floor panels, walls and partitions, elements of greenhouses, greenhouses, silos, reinforced concrete trays (L-3, L-4, L-5), elements of prefabricated reinforced concrete wells. For land reclamation systems, prefabricated concrete and reinforced concrete products are manufactured, such as pipes for pressure pipelines with prestressed fittings, smooth free-flow pipes, reinforced concrete trays (L-4, L-5, L-6), reinforced concrete rings and covers, reinforced concrete slabs (lay in open irrigation canals). In urban sewerage systems, reinforced concrete non-pressure socket pipes, non-pressure smooth pipes, reinforced concrete rings and covers are also used. Elements of prefabricated reinforced concrete fences are used to equip the fencing of important industrial facilities (nuclear power plants, military plants and ranges, etc.). When laying overhead power lines and communications, reinforced concrete poles of various shapes are widely used - round, square, rectangular, the same products are also used in the electrification of railways. Author: Alekseev V.S.
▪ General hygiene. Lecture notes ▪ Criminal executive law. Crib ▪ State and municipal finance. Crib
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ section of the site Mobile communications. Article selection ▪ article Tax press. Popular expression ▪ article Do raccoons wash their food? Detailed answer ▪ article Impatiens. Legends, cultivation, methods of application ▪ article Synchronous AC motors. Encyclopedia of radio electronics and electrical engineering ▪ article QRP CW transmitter. Encyclopedia of radio electronics and electrical engineering
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

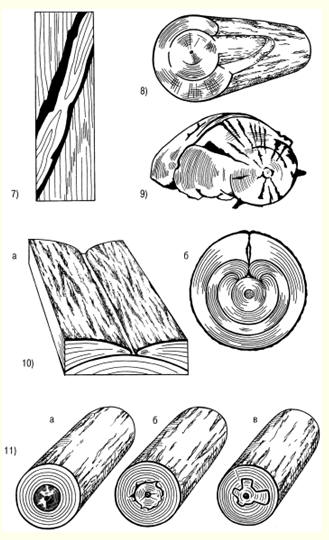
 See other articles Section
See other articles Section 