
|
|
Lecture notes, cheat sheets
Inorganic chemistry. Cheat sheet: briefly, the most important
Directory / Lecture notes, cheat sheets Table of contents
1. The relationship between the processes of metabolism and energy in the body. Thermodynamic system Life processes on Earth are largely due to the accumulation of solar energy in biogenic substances (proteins, fats, carbohydrates) and subsequent transformations of these substances in living organisms with the release of energy. The works of A. M. Lavoisier (1743-1794) and P. S. Laplace (1749-1827) showed by direct calorimetric measurements that the energy released in the process of life is determined by the oxidation of food products by oxygen in the air inhaled by animals. With the development in the XIX-XX centuries. thermodynamics, it became possible to quantitatively calculate the conversion of energy in biochemical reactions and predict their direction. The thermodynamic method is based on a number of strict concepts: "system", "state of the system", "internal energy of the system", "function of the state of the system". thermodynamic system any object of nature is called, consisting of a sufficiently large number of molecules (structural units) and separated from other objects of nature by a real or imaginary boundary surface (interface). Objects of nature that are not included in the system are called the environment. The most common characteristics of systems are m - the mass of the substance contained in the system, and E - the internal energy of the system. The mass of the substance of the system is determined by the total mass of the molecules of which it consists. The internal energy of the system is the sum of the energies of the thermal motion of molecules and the energy of interaction between them. Systems according to the nature of the exchange of matter and energy with the environment are divided into three types: isolated, closed and open. isolated system a system is called that does not exchange matter or energy with the medium (Δm = 0, ΔE = 0). A closed system is such a system that does not exchange matter with the environment, but can exchange energy (Δm = 0, ΔE^ 0). The exchange of energy can be carried out by transferring heat or doing work. open system such a system is called that can exchange both matter and energy with the medium (Δm ≠ 0, ΔE ≠ 0). An important example of an open system is the living cell. Systems, depending on the state of aggregation of the substance of which they are composed, are divided into homogeneous and heterogeneous. In a homogeneous system, there are no sharp changes in physical and chemical properties when moving from one area of the system to another. An example of such a system is blood plasma, which is a solution of various biogenic substances. A heterogeneous system consists of two or more homogeneous parts. An example of a heterogeneous system is whole blood, that is, plasma with cells - erythrocytes and leukocytes. 2. The first law of thermodynamics. Concepts characterizing the system The first law of thermodynamics provides a rigorous quantitative framework for analyzing the energy of various systems. To formulate it, it is necessary to introduce a number of new concepts that characterize the system. One of the most important concepts is the state of the system. A state is understood as a set of system properties that make it possible to define a system from the point of view of thermodynamics. As a generalized characteristic of the state of the system, the following concepts are used: "equilibrium", "stationary", "transitional state". The state of the system is called equilibrium if all properties remain constant for any long period of time and there are no flows of matter and energy in the system. If the properties of the system are constant in time, but there are flows of matter and energy, the state is called stationary. Quantitatively, the states are distinguished with the help of thermodynamic variables. Thermodynamic variables are those quantities that characterize the state of the system as a whole. They are also called thermodynamic parameters of the system. The most important thermodynamic variables are pressure p, temperature T, volume of the system V or the total mass of the system m, masses of chemicals (components) mk that make up the system, or the concentration of these substances m. It should be noted that similar characteristics (temperature, weight, composition of biological fluids, blood pressure) are used by the doctor to determine the patient's condition. The transition of a system from one state to another is called by process. As a result of the process, the state of the system and thermodynamic variables change. If we denote the value of the thermodynamic variable in the initial state as Х1 , and finally - X2 , then the change in this variable is respectively equal to ΔX = X2 - X1 and is called the increment of the thermodynamic variable X. The increment, taken with the opposite sign, is called the decrease in the variable X. The internal energy of the system E is one of the thermodynamic state functions. An important feature of state functions is their independence from the method of achieving a given state of the system. The change in the internal energy of the system ΔE is due to the work W, which is performed during the interaction of the system with the environment, and the exchange of heat Q between the environment and the system, the ratio between these quantities is the content of the first law of thermodynamics. The increase in the internal energy of the system ΔE in some process is equal to the heat Q received by the system, plus the work W done on the system in this process: ∆E=Q+W. In biological systems, heat is usually given off by the system to the external environment, and work is done by the system due to the loss of internal energy. It is convenient to represent the mathematical record of the first law of thermodynamics in the form: ∆E = Q - W. All quantities in the above formulas are measured in joules (J). 3. First law of thermodynamics The first law of thermodynamics is one of the fundamental laws of nature that cannot be derived from any other laws. Its validity is proved by numerous experiments, in particular, unsuccessful attempts to build a perpetual motion machine of the first kind, i.e., such a machine that could do work for an arbitrarily long time without supplying energy from outside. Depending on the conditions of the process in the system, various state functions are used, which are derived from the first law of thermodynamics. At the same time, instead of complex biological systems, simplified models are used to obtain conclusions about the transformations of mass and energy. The pressure in the system is maintained constant, it is equal to the external pressure. Such processes occurring at p = const are called isobaric. The expansion work done in an isobaric process is known to be: W = ρΔV, where ΔV is the volume increment of the system, equal to the difference between the volumes in states 2 and 1. Substituting the work of the expansion into the mathematical expression of the first law and carrying out simple transformations, we obtain: Qρ = ∆E + p∆V = (E2 + ρV2)-(E1 +ρΔV1) where Qρ is the heat of the isobaric process; 1, 2 - indexes related to the beginning and end of the process. The value (E + pV) is a function of the state of the system, denoted by H and called enthalpy: H = E + ρV. Accordingly, the expression can be written as: Qp = H2 - N1 = ∆H. From this expression it follows that enthalpy - state function, the increment of which is equal to the heat received by the system in the isobaric process. The measurement of the enthalpy increment in a certain process can be carried out by carrying out this process in a calorimeter at constant pressure. This is how A. M. Lavoisier and P. S. Laplace carried out their experiments, studying the energetics of metabolism in a living organism. In cases where the change in the state of the system occurs at a constant volume, the process is called isochoric. In this case, the volume change AV is equal to zero, and in accordance with the formula, the expansion work W = 0. Then from the mathematical expression of the first law of thermodynamics it follows: Qv = ∆E. The thermodynamic definition follows from the above relation: internal energy - state function, the increment of which is equal to the heat QV obtained by the system in the isochoric process. Therefore, the change in internal energy in a certain process can be measured by carrying out this process in a calorimeter at constant volume. It follows that at ρ = const the increments of internal energy and enthalpy are related by the relation: ∆H = ∆E + ρ∆V. 4. Hess' law The section of thermodynamics that studies the transformation of energy in chemical reactions is called chemical thermodynamics. The reaction equation for which the changes in internal energy ΔE, enthalpy ΔH or some other state function corresponding to this reaction are indicated is called thermochemical. Chemical reactions during which the enthalpy of the system decreases (ΔH < 0) and heat is released into the external environment are called exothermic. Reactions in which the enthalpy increases (ΔH > 0) and the system absorbs heat Qp outside are called endothermic. The oxidation of glucose with oxygen occurs with the release of a large amount of heat (Qp \u2800d XNUMX kJ / / mol), i.e. this process is exothermic. The corresponding thermochemical equation can be written as С6 Н12 О6 + 602 = 6С02 + 6Н2Oh, ΔH = 2800 kJ. Reactions occurring in solution are usually accompanied by a slight change in the volume of the system, i.e., ΔV ≈ 0. In this regard, in many cases, in biological calculations, we can assume that ΔH = ΔE. Consequently, the release of heat in such systems is mainly due to a decrease in internal energy as a result of the reaction, and vice versa. The enthalpy of formation of compound A is the change in the enthalpy of the system ΔHA accompanying the formation of 1 mol of compound A from simple substances. The enthalpies of formation of oxygen, carbon, hydrogen and all other elemental (simple) substances are assumed to be zero. Other things being equal, internal energy and enthalpy are proportional to the amount of matter in the system. Such thermodynamic functions are called extensive. From the point of view of thermodynamics, the reaction of the general form nAA + pВ = nС + nD , Δh is the transition of the system from the initial state with the enthalpy H1 to state 2 with enthalpy H2. The change in the enthalpy of the system as a result of this transition, called the enthalpy of this reaction, is equal to the difference: ΔHpya = H2 - N1 = (nocHc + nDHD) - (nАНА + nBHB). The law of constancy of heat sums was discovered by the Russian chemist G. I. Hess in 1840. He is the discoverer of the applicability of the first law of thermodynamics in chemical transformations and the founder of chemical thermodynamics. Currently, Hess's law is considered as a consequence of the first law of thermodynamics and is formulated as follows: the enthalpy increment in the formation of given products from given reagents at constant pressure does not depend on the number and type of reactions resulting in the formation of these products. In thermochemical calculations, it is not the Hess law itself that is more often used, but its consequence, derived above for the particular case of glucose oxidation in the form of equality (2). For a reaction presented in general form pАA + pвB = = nсC + nDD, a consequence of the Hess law is written using the equality ΔHpya = (noCΔHC + nDΔHD) - (nAΔHA ++nBΔHB ) and is formulated as follows: the enthalpy of reaction is equal to the algebraic sum of the enthalpies of formation of a stoichiometric amount of products minus the algebraic sum of the enthalpies of formation of a stoichiometric amount of reactants. 5. The second law of thermodynamics. Gibbs free energy The body performs work by expending internal energy stored in the form of the energy of chemical interaction of atoms of its constituent substances. The mathematical expression ΔE \uXNUMXd Q - W of the first law of thermodynamics determines the exact relationship between the consumption of internal energy of the system ΔЕ, the work W performed by the system, and the energy Q, which is lost in the form of heat. However, from the first law of thermodynamics it is impossible to determine the part of the expended internal energy that can be converted into work. Theoretical cost estimates are based on the second law of thermodynamics. This law imposes strict restrictions on the efficiency of converting energy into work and, in addition, allows you to introduce criteria for the possibility of a spontaneous flow of a process. The process is called spontaneousif it is carried out without any influences, when the system is left to itself. There are processes in which the internal energy of the system does not change (ΔE = 0). Such processes include, for example, the ionization of acetic acid in water. A number of spontaneous processes proceed with an increase in internal energy (ΔE > 0). This includes, in particular, typical reactions of the formation of bioinorganic compounds of albumin (blood plasma protein) with metal ions, such as Cu2+. The change in internal energy AE for closed systems cannot serve as a criterion for spontaneous processes. Consequently, the first law of thermodynamics, from which this criterion is derived, is not enough to solve the question of spontaneity, as well as the efficiency of processes. The solution of these questions is achieved with the help of the second law of thermodynamics. To formulate the second law of thermodynamics, it is necessary to introduce the concepts of reversible and irreversible processes in the thermodynamic sense. If the system is in equilibrium, this state is maintained indefinitely under the same external conditions. When external conditions change, the state of the system can change, i.e., a process can take place in the system. A process is said to be thermodynamically reversible if, during the transition from the initial state 1 to the final state 2, all intermediate states are in equilibrium. A process is called thermodynamically irreversible if at least one of the intermediate states is nonequilibrium. A reversible process can be carried out only with a sufficiently slow change in the parameters of the system - temperature, pressure, concentration of substances, etc. The rate of change in the parameters should be such that the deviations from equilibrium that occur during the process are negligible. It should be noted that an important problem in medicine is associated with reversibility - the preservation of tissues at low temperatures. Reversible processes are the limiting case of real processes occurring in nature and carried out in industry or in laboratories. 6. The second law of thermodynamics. Entropy Maximum work Wmax, which can be obtained with a given loss of internal energy ΔE in the process of transition from state 1 to state 2, is achieved only if this process is reversible. In accordance with the expression for the first law of thermodynamics, the minimum heat Qmin Qmin \uXNUMXd ΔE - Wmax . The maximum achievable efficiency factor, which characterizes the cost efficiency of the internal energy of the system, is respectively equal to ηmax=Wmax / ΔE. In an irreversible process of transition from state 1 to state 2, the work done by the system is less than W. To calculate the maximum factor hmax with a known value of ΔE, it is necessary to know the value of Wmax or Qmin Wmax = ΔE - Qmin , therefore, ηmax \u1d XNUMX - ΔE / Qmin . Q valuemin can be calculated from the second law of thermodynamics using a thermodynamic state function called entropy. The concept of entropy was introduced (1865) by the German physicist R. Yu. Clausius (1822-1888), one of the founders of thermodynamics and the molecular kinetic theory of thermal processes. Thermodynamic definition of entropy according to Clausius: entropy is a state function whose increment ΔS is equal to heat Qmin brought to the system in a reversible isothermal process, divided by the absolute temperature T at which the process is carried out: ∆S = Qmin / T. It follows from the formula that the unit of entropy is J/K. An example of a reversible isothermal process is the slow melting of ice in a thermos filled with water at 273°K. It has been experimentally established that for the melting of 1 mole of ice (18 g) it is necessary to supply at least 6000 J of heat. In this case, the entropy of the "ice - water" system in the thermos increases by ΔS = 6000 J: 273°K = 22 J/K. When a thermos with water is cooled at 273°K, 6000 J of heat can be slowly removed, and 1 mol of ice is formed during the crystallization of water. For this process, Qmin in the formula is negative. Accordingly, the entropy of the "ice - water" system during the formation of 1 mole of ice decreases by ΔS = 22 J/K. Similarly, it is possible to calculate the change in entropy for any isothermal physical and chemical processes, if the heat supplied to the system or removed from it during these processes is known. As is known from physics, this heat can be determined using calorimetric measurements. Thus, the change in entropy, as well as in two other functions of the state of the system - internal energy and enthalpy, is an experimentally determined quantity. The physical meaning of entropy, as well as internal energy, is clearly revealed when considering processes occurring in isolated systems from the molecular-kinetic point of view. 7. Boltzmann formula Isolated systems, by definition, do not exchange either matter or energy with the environment. Of course, such systems do not really exist in nature. However, very good insulation can be achieved by placing the system in a thermos sealed with a cork. It turns out that any spontaneous process can take place in an isolated system only if it is characterized by an increase in entropy; in equilibrium, the entropy of the system is constant: ∆S ≥ 0. This statement, based on experimental observations, is one of the possible formulations of the second law of thermodynamics. A process that is inverse to spontaneous, according to the second law of thermodynamics, cannot proceed in an isolated system, since such a process is characterized by a decrease in entropy. An examination of various isolated systems shows that spontaneous processes are always associated with an increase in the number of microstates w of the system. In the same processes, the entropy S of the system increases, i.e., the entropy increases with an increase in the number of microstates. For the first time, the existence of such a dependence was noticed by the Austrian physicist L. Boltzmann, who in 1872 proposed the relationship: КБ =R/NA = 1,38 - 1023 J/K, where KБ - Boltzmann constant, equal to the ratio of the gas constant R to the Avogadro constant NA . This relation is called the Boltzmann formula. The Boltzmann formula makes it possible to theoretically calculate the entropy of a system from the number of its possible microstates. Such calculations are in good agreement with the experimentally determined values. In particular, it is known that the number of microstates of crystalline substances at 0°K is close to w0 "1. Thus, the absolute values of the entropy of crystallizing substances can be determined, in contrast to the internal energy E and enthalpy H, for which only relative values can be determined. An increase in the number of microstates of a system in many cases can be associated with an increase in disorder in this system, with a transition to more probable distributions of the system's energy. Based on the Boltzmann relation, one can give a molecular-kinetic definition of entropy. Entropy is a measure of the probability of a system being in a given state or a measure of the disorder of the system. The importance of the concept of entropy is due to the fact that on the basis of this value it is possible to predict the direction of spontaneous processes. However, the applicability of measuring entropy as a criterion for the direction of processes is limited to isolated systems in accordance with the formulation of the second law of thermodynamics. 8. Gibbs energy A new state function, the Gibbs energy, is introduced as a criterion for the spontaneity of processes in open and closed systems. This function was named after the great American physicist DW Gibbs (1839-1903), who derived this function and then used it in thermodynamic work. The Gibbs energy is determined in terms of the enthalpy H and the entropy S using the relationships: G = H - S, ∆G = ∆H - ∆S. Based on the Gibbs energy, the second law of thermodynamics can be formulated as follows: under isobaric isothermal conditions (p, T = const), only such processes can spontaneously occur in the system, as a result of which the Gibbs energy of the system decreases (ΔG < 0). In the state of equilibrium, the Gibbs energy of the system does not change (G = const, AG = 0). ΔG < 0, p, T = const. It follows from the foregoing that the Gibbs energy plays an important role in the study of bioenergetic processes. With this state function, you can predict the direction of spontaneous processes in biological systems and calculate the maximum achievable efficiency. The Gibbs energy G, like the enthalpy H, is a function of the state of the system. Therefore, the change in the Gibbs energy ΔG can be used to characterize chemical transformations in a similar way to the change in enthalpy ΔH. The reaction equations for which the Gibbs energy change corresponding to these reactions is indicated are also called thermochemical. Chemical reactions, during which the Gibbs energy of the system decreases (ΔG < 0) and work is done, are called exergonic. Reactions, as a result of which the Gibbs energy increases (ΔG > 0) and work is done on the system, are called endergonic. Derived from the second law of thermodynamics, the Gibbs energy is a function of state. Therefore, just as for enthalpy, the Hess law for the Gibbs energy can be formulated in the following form: the change in the Gibbs energy during the formation of given products from given reagents at constant pressure and temperature does not depend on the number and type of reactions resulting in the formation of these products. An important example of the application of Hess's law is the calculation of the Gibbs energy of the reaction of glucose oxidation with dioxygen. The change in the Gibbs energy in this reaction at p = 101 kPa and T = 298°K, determined outside the body, is ΔG° = 2880 kJ/mol. The corresponding thermochemical equation is written as: С6Н12О6 + 6O2 = 6CO2 + 6Н2Oh, ΔGpya° = 2880 kJ/mol. In the cells of the body, this reaction is carried out through a number of successive stages studied by biochemists. It can be predicted from Hess' law that the sum of the Gibbs energy changes in all intermediate reactions is ΔGpya: ΔG1 +ΔG2 +ΔG3 + … + ∆Gn = ∆Gpya °. The Gibbs energy of a reaction is equal to the algebraic sum of the Gibbs energies of formation of a stoichiometric amount of products minus the algebraic sum of the Gibbs energies of formation of a stoichiometric amount of reactants: ΔGpya = (nocΔGc + nDΔGD)(nAΔGA + nBΔGB). 9. Solutions. Classification of solutions According to the state of aggregation, solutions can be gaseous, liquid and solid. Any solution consists of solutes and a solvent, although these concepts are somewhat arbitrary. For example, depending on the ratio of the amount of alcohol and water, this system can be a solution of alcohol in water or water in alcohol. Usually, the solvent is considered to be the component that is in the solution in the same state of aggregation as before dissolution. The doctrine of solutions is of particular interest to physicians because the most important biological fluids - blood, lymph, urine, saliva, sweat - are solutions of salts, proteins, carbohydrates, lipids in water. Biological fluids are involved in the transport of nutrients (fats, amino acids, oxygen), drugs to organs and tissues, as well as in the excretion of metabolites (urea, bilirubin, carbon dioxide, etc.) from the body. Blood plasma is a medium for cells - lymphocytes, erythrocytes, platelets. In the liquid media of the body, the constancy of acidity, the concentration of salts and organic substances is maintained. This constancy is called concentration homeostasis. Classification of solutions Solutions of substances with a molar mass of less than 5000 g/mol are called solutions of low molecular weight compounds (NMS), and solutions of substances with a molar mass of more than 5000 g/mol are called solutions of high molecular weight compounds (HMC). According to the presence or absence of electrolytic dissociation, NMS solutions are divided into two classes - solutions of electrolytes and non-electrolytes. Electrolyte solutions - solutions of salts, acids, bases, ampholytes dissociating into ions. For example, KNO solutions3, HCl, KOH, Al(OH)3 , glycine. The electrical conductivity of electrolyte solutions is higher than that of the solvent. Solutions of non-electrolytes - solutions of substances that practically do not dissociate in water. For example, solutions of sucrose, glucose, urea. The electrical conductivity of non-electrolyte solutions differs little from that of a solvent. Solutions of NMS (electrolytes and non-electrolytes) are called true, in contrast to colloidal solutions. True solutions are characterized by a homogeneous composition and the absence of an interface between the solute and the solvent. The size of dissolved particles (ions and molecules) is less than 109м. Most IUDs are polymers whose molecules (macromolecules) consist of a large number of repeating groups or monomeric units interconnected by chemical bonds. IUD solutions are called polyelectrolyte solutions. Polyelectrolytes include polyacids (heparin, polyadenylic acid, polyaspartic acid, etc.), polybases (polylysine), polyampholytes (proteins, nucleic acids). The properties of HMS solutions differ significantly from those of NMS solutions. Therefore, they will be discussed in a separate section. This chapter is devoted to solutions of low molecular weight electrolytes, ampholytes and non-electrolytes. 10. Water as a solvent The most common solvent on our planet is water. The body of an average person weighing 70 kg contains approximately 40 kg of water. At the same time, about 25 kg of water falls on the liquid inside the cells, and 15 kg is extracellular fluid, which includes blood plasma, intercellular fluid, cerebrospinal fluid, intraocular fluid and liquid contents of the gastrointestinal tract. In animal and plant organisms, water is usually more than 50%, and in some cases the water content reaches 90-95%. Due to its anomalous properties, water is a unique solvent, perfectly adapted for life. First of all, water dissolves ionic and many polar compounds well. This property of water is associated to a large extent with its high dielectric constant (78,5). Another large class of substances that are highly soluble in water includes such polar organic compounds as sugars, aldehydes, ketones, and alcohols. Their solubility in water is explained by the tendency of water molecules to form polar bonds with the polar functional groups of these substances, for example, with the hydroxyl groups of alcohols and sugars or with the oxygen atom of the carbonyl group of aldehydes and ketones. The following are examples of hydrogen bonds important for the solubility of substances in biological systems. Due to its high polarity, water causes the hydrolysis of substances. Since water is the main part of the internal environment of the body, it provides the processes of absorption, movement of nutrients and metabolic products in the body. It should be noted that water is the end product of the biological oxidation of substances, in particular glucose. The formation of water as a result of these processes is accompanied by the release of a large amount of energy - approximately 29 kJ / mol. Other anomalous properties of water are also important: high surface tension, low viscosity, high melting and boiling points, and a higher density in the liquid state than in the solid state. Water is characterized by the presence of associates - groups of molecules connected by hydrogen bonds. Depending on their affinity for water, the functional groups of dissolved particles are divided into hydrophilic (attracting water), easily solvated by water, hydrophobic (repelling water), and amphiphilic. Hydrophilic groups include polar functional groups: hydroxyl -OH, amino -NH2 , thiol -SH, carboxyl -COOH. To hydrophobic - non-polar groups, for example hydrocarbon radicals: CHXNUMX-(CH2)п -, FROM6Н5 -. Amino acids include substances (amino acids, proteins) whose molecules contain both hydrophilic groups (-OH, -NH2 , -SH, -COOH) and hydrophobic groups: (CH3 - (CH2)п ,-FROM6Н5-). When amphiphilic substances are dissolved, the structure of water changes as a result of interaction with hydrophobic groups. The degree of ordering of water molecules close to hydrophobic groups increases, and the contact of water molecules with hydrophobic groups is minimized. Hydrophobic groups, associating, push water molecules out of their area of location. 11. The concentration of the solution and how to express it Solution A homogeneous system of variable composition of two or more substances in a state of equilibrium is called. The substances that make up a solution are called solution components. An important characteristic of a solution is its concentration. This value determines many properties of the solution. Substance concentration (solution component) is a quantity measured by the amount of a solute contained in a certain mass or volume of a solution or solvent. The most commonly used ways of expressing concentration are: mass fraction, molar concentration, molar equivalent concentration, mole fraction, volume fraction, titer. Mass fraction W(X) expressed in fractions of a unit, percent (%), ppm (a thousandth of a percent) and in parts per million (ppm). The mass fraction is calculated by the formulas: W(X) = m(X)/m(Pp), W(X) = m(X)/m(Pp) × 100%, where m(X) - mass of the given component X (solute), kg (g); m(Pp) is the mass of the solution, kg (g). Molar concentration is expressed in mol/m3 , mol/dm3 , mol/cm3 , mol/l, mol/ml. In medicine, the use of units of mol / l is preferable. The molar concentration is calculated by the formula: C(X) = n(X)/V(pp) = m(X)/M(X) ×V(rr), where n(X) - amount of the dissolved substance of the system, mol; M(X) is the molar mass of the solute, kg/mol or g/mol; m(X) is the mass of the dissolved substance, respectively, kg or g; V(rr) - volume of solution, l. molar concentration b(X) expressed in units of mol/kg. Recording form, for example: b (HCl) \u0,1d XNUMX mol / kg. Calculate the molar concentration by the formula: b(X) = n(X)/m(rl) = m(X)/M(X) ×m(rl) where m(rl) - mass of solvent, kg. In chemistry, the concept of equivalent and equivalence factor is widely used. Equivalent a real or conditional particle of substance X is called, which in a given acid-base reaction is equivalent to one hydrogen ion, or in a given redox reaction - to one electron, or in a given exchange reaction between salts - to a unit of charge. Volume fraction f(X) expressed in fractions of a unit or as a percentage, it is calculated by the formula: Ф(X) =V(X)/ V(rr) where v(X) - the volume of this component X of the solution; V(rr) is the total volume of the solvent. The titer of the solution is denoted by T(X), unit of measurement - kg/cm3 , g/cm3 , g/ml. The titer of the solution can be calculated using the formula: Т(X) = m(X)/ V(rr) where m(X) is the mass of the substance, usually g; V(rr) solution volume, ml. 12. Dissolution process The nature of the dissolution process is complex. Naturally, the question arises why some substances are easily soluble in some solvents and poorly soluble or practically insoluble in others. The formation of solutions is always associated with certain physical processes. One such process is the diffusion of a solute and a solvent. Due to diffusion, particles (molecules, ions) are removed from the surface of the dissolved substance and are evenly distributed throughout the entire volume of the solvent. That is why, in the absence of stirring, the dissolution rate depends on the diffusion rate. However, it is impossible to explain the unequal solubility of substances in various solvents only by physical processes. The great Russian chemist D. I. Mendeleev (1834-1907) believed that chemical processes play an important role in dissolution. He proved the existence of sulfuric acid hydrates H2Total sq4H2O,H2Total sq42H2O,H2Total sq44H2O and some other substances, for example, C2Н5OH3H2A. In these cases, the dissolution is accompanied by the formation of chemical bonds between the particles of the solute and the solvent. This process is called solvation, in the particular case when the solvent is water, hydration. As established, depending on the nature of the solute, solvates (hydrates) can be formed as a result of physical interactions: ion-dipole interaction (for example, when dissolving substances with an ionic structure (NaCI, etc.); dipole-dipole interaction - when dissolving substances with a molecular structure (organic substances )). Chemical interactions are carried out due to donor-acceptor bonds. Here, the solute ions are electron acceptors, and the solvents (Н2Oh, NH3) - electron donors (for example, the formation of aqua complexes), as well as as a result of the formation of hydrogen bonds (for example, the dissolution of alcohol in water). Evidence for the chemical interaction of a solute with a solvent is provided by the thermal effects and the color change that accompanies the dissolution. For example, when potassium hydroxide is dissolved in water, heat is released: KOH + xN2O \uXNUMXd KOH (N2Oh; ΔH°solution = 55 kJ/mol. And when sodium chloride is dissolved, heat is absorbed: NaCI + xH2O = NaCl(H2Oh; ΔH°solution = +3,8 kJ/mol. The heat released or absorbed when 1 mole of a substance is dissolved is called heat of solution Qsolution According to the first law of thermodynamics Qsolution = ΔHsolution, where ΔHsolution is the change in enthalpy upon dissolution of a given amount of a substance. Dissolution of anhydrous white copper sulfate in water leads to the appearance of an intense blue color. The formation of solvates, color change, thermal effects, as well as a number of other factors, indicate a change in the chemical nature of the components of the solution during its formation. Thus, in accordance with modern concepts, dissolution is a physicochemical process in which both physical and chemical types of interaction play a role. 13. Thermodynamics of the dissolution process According to the second law of thermodynamics, at p, T = const, substances can spontaneously dissolve in any solvent if, as a result of this process, the Gibbs energy of the system decreases, i.e. ΔG = (ΔН - TΔS) < 0. The value of ΔН is called the enthalpy factor, and the value of TΔS is called the entropy factor of dissolution. When liquid and solid substances are dissolved, the entropy of the system usually increases (ΔS > 0), since the dissolved substances pass from a more ordered state to a less ordered one. The contribution of the entropy factor, which contributes to the increase in solubility, is especially noticeable at elevated temperatures, because in this case the factor T is large and the absolute value of the product TΔS is also large, respectively, the decrease in the Gibbs energy increases. When dissolving gases in a liquid, the entropy of the system usually decreases (ΔS < 0), since the solute from a less ordered state (large volume) passes into a more ordered state (small volume). A decrease in temperature favors the dissolution of gases, because in this case the factor T is small and the absolute value of the product TΔS will be the smaller, and the decrease in the Gibbs energy will be greater, the lower the value of T. During the formation of a solution, the enthalpy of the system can also both increase (NaCl) and decrease (KOH). The change in the enthalpy of the dissolution process must be considered in accordance with the Hess law as the algebraic sum of the endo and exothermic contributions of all processes accompanying the dissolution process. These are the endothermic effects of the destruction of the crystal lattice of substances, the breaking of the bonds of molecules, the destruction of the initial structure of the solvent, and the exothermic effects of the formation of various interaction products, including solvates. For simplicity of presentation, the increment of the enthalpy of dissolution ΔНsolution can be represented as the energy difference Ecr, spent on the destruction of the crystal lattice of the dissolved substance, and energy ESol, released during the solvation of solute particles by solvent molecules. In other words, the enthalpy change is the algebraic sum of the enthalpy change ΔHcr as a result of the destruction of the crystal lattice and a change in the enthalpy ΔНSol due to solvation by solvent particles: ΔNsolution = ΔHcr + ΔHSol, where ΔHsolution - enthalpy change during dissolution. However, the dissolution of noble gases in organic solvents is often accompanied by the absorption of heat, for example, helium and neon in acetone, benzene, ethanol, and cyclohexane. When dissolving solids with a molecular crystal structure and liquids, molecular bonds are not very strong, and therefore usually ΔHSol > ΔNcr This leads to the fact that the dissolution of, for example, alcohols and sugars is an exothermic process (ΔHsolution < 0). When dissolving solids with an ionic lattice, the energy ratio Ecr and ESol may be different. However, in most cases, the energy released during the solvation of ions does not compensate for the energy spent on the destruction of the crystal lattice; therefore, the dissolution process is endothermic. Thus, thermodynamic data make it possible to predict the spontaneous dissolution of various substances based on the first and second laws of thermodynamics. 14. Solubility If a solute is in contact with a solvent, the process of solution formation in many cases proceeds spontaneously until a certain limiting concentration is reached (saturation occurs). This happens when equilibrium is reached, when the enthalpy and entropy factors are equal, i.e. ΔН = TΔS. For example, when crystals are introduced into a liquid, molecules or ions pass from the surface of the crystal into the solution. Due to diffusion, the particles are evenly distributed throughout the volume of the solvent. The dissolution proceeds to saturation. A solution that contains the maximum amount of solute at a given temperature and is in equilibrium with an excess of solute is called saturated. A supersaturated solution is a solution whose concentration is higher than that of a saturated solution. A solution with a lower concentration than a saturated solution is called unsaturated. The ability of a substance to dissolve in a particular solvent is called solubility. Numerically, the solubility of a substance is equal to the concentration of its saturated solution. Solubility can be expressed in the same units as concentration, for example, in terms of the amount of solute contained in 1 liter of a saturated solution, mol/l, or in terms of the mass of a solute in 100 g of a saturated solution. The unit of solubility is grams per 100 g of solvent. The corresponding value is called the solubility coefficient. Solubility depends on the nature of the solute and solvent, temperature, pressure, and the presence of other substances in the solution. Influence on the solubility of the nature of the components The ability of substances to dissolve is determined by the nature of the forces of interaction between the molecules of the components of the solution X1 them2 : solvent - solvent (X1 - X1 ), solute - solute (X2 - X2 ), solvent - solute (X1 - X2 ) (points indicate molecular bond). The solubility of substances varies widely. The examples show the solubility of different salts in the same solvent (water) and the solubility of the same substance (AgNO3 ) in various solvents. Substances with an ionic type of bond and substances consisting of polar molecules dissolve better in polar solvents, such as water, alcohols. These solvents are characterized by high dielectric constant. The high solubility of substances is quite often due to the formation of intermolecular, in particular hydrogen, bonds. Thus, the unlimited mutual solubility of water and alcohol is explained by the formation of hydrogen bonds between water and alcohol molecules, and the dissolution of AgcI crystals in an aqueous solution of ammonia is explained by the formation of a chemical donor-acceptor bond of a silver ion with ammonia molecules (AgCI is practically insoluble in water). For the same reason, pyridine, a solvent with a low permittivity, exhibits a very high solubility. Since solubility characterizes true equilibrium, the influence of external conditions on this state (pressure, temperature) can be qualitatively estimated using the Le Chatelier principle. Such assessments are necessary in the practice of deep diving, when working in hot shops, etc. 15. Solubility of gases in liquids. Laws of Henry-Dalton and Sechenov The dissolution of gases in liquids is almost always accompanied by the release of heat. Therefore, the solubility of gases decreases with increasing temperature according to Le Chatelier's principle. This pattern is often used to remove dissolved gases from water (eg CO02) by boiling. Sometimes the dissolution of a gas is accompanied by the absorption of heat (for example, the dissolution of noble gases in some organic solvents). In this case, increasing the temperature increases the solubility of the gas. A gas does not dissolve in a liquid indefinitely. At a certain gas concentration X, an equilibrium is established:  When a gas dissolves in a liquid, a significant decrease in the volume of the system occurs. Therefore, an increase in pressure, according to Le Chatelier's principle, should lead to a shift of the equilibrium to the right, i.e., to an increase in the solubility of the gas. If the gas is slightly soluble in a given liquid and the pressure is low, then the solubility of the gas is proportional to its pressure. This dependence is expressed by Henry's law (1803): the amount of gas dissolved at a given temperature in a certain volume of liquid at equilibrium is directly proportional to the pressure of the gas. Henry's law can be written in the following form: с (X) =Kr(X) ×P(X) where is the concentration of gas in a saturated solution, mol/l; P(X) - gas pressure X over the solution, Pa; Kr(X) - Henry's constant for gas X, mol × l1 × Pa1 . Henry's constant depends on the nature of the gas, solvent, and temperature. Henry's law is valid only for relatively dilute solutions, at low pressures and in the absence of chemical interaction between the molecules of the dissolved gas and the solvent. Henry's law is a special case of the general Dalton's law. If we are talking about the dissolution of not one gaseous substance, but a mixture of gases, then the solubility of each component obeys Dalton's law: the solubility of each of the components of the gas mixture at a constant temperature is proportional to the partial pressure of the component above the liquid and does not depend on the total pressure of the mixture and the individuality of other components. In other words, in the case of the dissolution of a mixture of gases in a liquid, the partial pressure p! this component. The partial pressure of a component is understood as the proportion of the pressure of the component from the total pressure of the gas mixture: Рi/ Rcommon The partial pressure of the component is calculated by the formula Studying the solubility of gases in liquids in the presence of electrolytes, the Russian physiologist I. M. Sechenov (1829-1905) established the following pattern (Sechenov's law): the solubility of gases in liquids in the presence of electrolytes decreases; gases are released. Рi = Pcommon ×(Xi) where pi - partial pressure of component Xi; Рcommon total pressure of the gas mixture; x(Xi) is the mole fraction of the ith component. Studying the solubility of gases in liquids in the presence of electrolytes, the Russian physiologist I. M. Sechenov (1829-1905) established the following pattern (Sechenov's law): the solubility of gases in liquids in the presence of electrolytes decreases; gases are released. 16. The role of diffusion in the processes of transfer of substances in biological systems Diffusion plays an important role in biological systems. First of all, the movement of nutrients and metabolic products in tissue fluids occurs through diffusion. In addition, in many cases, the rate of physicochemical processes in living organisms is determined by the rate of diffusion of reactants, since diffusion of reactants is usually the slowest stage of the process, while biochemical reactions involving enzymes proceed very quickly. Every living cell is surrounded by a membrane that serves to protect and regulate the intracellular environment. Substances pass through membranes by two main mechanisms: by ordinary diffusion (passive transport) and by energetically activated transfer (active transport). The inner layer of the membrane consists of hydrocarbon chains. Therefore, many small neutral molecules and non-polar HMS molecules are soluble in this layer and can pass through the membrane by normal diffusion along a concentration gradient. Such transport of substances is called passive. Diffusion plays an important role in the process of oxygenation of blood in the lungs. Due to the large branching, the surface of the alveoli of the lungs is large (~ 80 m2), so oxygen is actively dissolved in the plasma and enters the erythrocytes. Venous blood is depleted of oxygen - the concentration of oxygen in venous blood tends to zero. Therefore, the oxygen concentration gradient between the atmosphere and the blood entering the lungs is high, resulting in active uptake (according to Fick's law). The movement of substances from an area of lower concentration to an area of higher concentration against a gradient is called active transport. Such a process cannot proceed spontaneously and requires energy costs. The energy source is the exoergonic hydrolysis reaction of a bioinorganic compound - adenosine triphosphate (ATP). A stable stationary distribution of K ion concentrations inside and outside the cell is achieved when the flow of K ions through the membrane into the cell becomes equal to the flow of K ions from the cell due to passive diffusion. Distribution (ion homeostasis) is similarly achieved for Na ions, only active transport and compensating passive diffusion of ions are directed opposite to the corresponding flows of K ions. The diffusion process is widely used in medicine. For example, the dialysis method based on the selectivity of diffusion of low molecular weight substances through a semipermeable membrane along a concentration gradient is used in clinical practice to create an "artificial kidney" apparatus. IUD particles do not pass through a semipermeable membrane, so biological fluids (for example, blood plasma) can be purified by dialysis from harmful low-molecular substances - "slags" (urea, uric acid, bilirubin, amines, excess K ions) that accumulate in various diseases. During purification, the patient's blood, drawn from the vein, enters special chambers with semi-permeable membranes, through which the NMS can diffuse and be removed from the plasma. In a number of inflammatory diseases, protein destruction occurs, and in the blood plasma, along with NMS, there are protein fragments (peptides and polypeptides) that also need to be removed. 17. Lowering the freezing point and raising the boiling point of solutions A direct consequence of the decrease in vapor pressure over the solution is a change in the freezing point ΔТз and boiling point of solutions ΔTк compared with the values of these quantities for a pure solvent. The relationships between these quantities also follow from the second law of thermodynamics. The boiling point of a liquid is the temperature at which its vapor pressure becomes equal to the external pressure (for example, at 101,3 kPa, the boiling point of water is 100 ° C). The freezing point (crystallization) of a liquid is the temperature at which the vapor pressure over the liquid is equal to the vapor pressure over the solid phase. If we designate the freezing and boiling points of the solution T3 and Tk, and the same values for the solvent T °3 and T°к , then we get: ΔTk = Tк - T°к > 0, ΔT3 = T°3 - T3 > 0. The effects of increasing the boiling point and lowering the freezing point of solutions can be qualitatively explained using Le Chatelier's principle. Indeed, if in an equilibrium system "liquid - vapor" (for example, H2О(H) - N2О(G)), introduce a soluble non-volatile substance, then the vapor pressure of the solvent over the solution will decrease. To compensate for the decrease in vapor pressure and achieve the previous equilibrium state, the solution must be heated to a higher temperature (more than 373°K), since the process is endothermic. Let there be an equilibrium system "solid phase - liquid", for example H2О(T) > H2О(H), at 273°K. If a certain amount of a non-volatile substance (insoluble in the solid phase) is dissolved in the liquid phase, then the concentration of water molecules in the liquid phase will decrease. In accordance with the principle of Le Chatelier, a process will begin to increase the amount of water in the liquid phase - the melting of ice. To establish a new equilibrium, the solution must be cooled, that is, the temperature must be lowered, since the process is exothermic. According to Raoult's law for dilute solutions, the decrease in vapor pressure is proportional to the concentration of the solution. Therefore, the increase in the boiling point and the decrease in the freezing point of such solutions should increase with increasing their concentration. Studying the freezing and boiling of solutions, Raul found: an increase in the boiling point ΔTк and lowering the freezing point ΔT3 dilute solutions of non-electrolytes is directly proportional to the molar concentration of the solution: ΔTк =Kэb(X), ΔT3 = Kз b(X), where b(X) - molar concentration, mol/kg; Кз and Kэ - coefficients of proportionality, kg × K × mol1 , which are called the ebulliometric and cryometric constants, respectively. The physical meaning of the constants Kэ and Kз becomes clear if we accept b(X) = 1. Then Kэ = ΔTк , and Kз = ΔTз . In other words, the ebulliometric constant is numerically equal to the increase in the boiling point of a one-molar solution, and the cryometric constant is numerically equal to the decrease in the freezing point of a one-molar solution. Ebuliometric and cryometric constants depend only on the nature of the solvent and do not depend on the nature of the solute (ideal solutions). 18. Osmotic pressure Osmosis is the predominantly unilateral penetration of solvent molecules (diffusion) through a semipermeable membrane from a solvent into a solution or from a solution with a lower concentration into a solution with a higher concentration. A necessary condition for the occurrence of osmosis is the presence of a solvent and a solution or two solutions of different concentrations, separated by a semipermeable membrane. From the point of view of thermodynamics, the driving force of osmosis is the tendency of the system to equalize concentrations, since in this case the entropy of the system increases, since the system passes into a less ordered state, the Gibbs energy of the system decreases accordingly, and the chemical potentials are equalized. Therefore, osmosis is a spontaneous process. A simple experiment can serve as an illustration explaining the connection between the mechanism of osmosis and a change in vapor pressure over a solution. If a beaker with a pure solvent and a beaker with a solution of some non-volatile substance (the levels of liquids in the vessels are the same) are placed in a closed glass vessel, then after a while the level of the liquid in the first beaker will decrease, and the level of the solution in the second beaker will rise. In this case, the solvent passes from the first beaker to the second beaker, which is due (according to Raoult's law) to a lower vapor pressure of the solvent over the solution than over the pure solvent. Thus, the air space between the solvent and the solution acts as a semi-permeable membrane. Let us fill a vessel with semi-permeable walls with an aqueous solution of glucose and place it in another vessel with water so that the levels of the liquids in these vessels coincide. As a result of osmosis, the volume of the solution in the first vessel increases and the liquid level in this vessel gradually rises. This creates an additional hydrostatic pressure that prevents osmosis. The hydrostatic pressure of the liquid column at osmotic equilibrium determines the osmotic pressure of the solution. Osmotic pressure called the value measured by the minimum hydraulic pressure that must be applied to the solution to stop osmosis. Laws of osmotic pressure. Van't Hoff proposed an empirical equation for calculating the osmotic pressure of dilute solutions of non-electrolytes: π = C(X)RT, where π - osmotic pressure, kPa; С(X) - molar concentration, mol/l; R is the universal gas constant equal to 8,31 kPa - l / (mol - K); T - absolute temperature, K. Although the van't Hoff law was established on the basis of experimental data, it can be derived from the conditions of thermodynamic equilibrium at ΔG = 0. Therefore, this law should be considered as a consequence of the second law of thermodynamics. The expression in the above form is similar to the Clapeyron-Mendeleev equation for ideal gases, however, these equations describe different processes. 19. The role of osmosis and osmotic pressure in biological systems The phenomenon of osmosis plays an important role in many chemical and biological systems. Osmosis regulates the flow of water into cells and intercellular structures. The elasticity of cells (turgor), which ensures the elasticity of tissues and the preservation of a certain shape of organs, is due to osmotic pressure. Animal and plant cells have shells or a surface layer of protoplasm that has the properties of semipermeable membranes. When these cells are placed in solutions with different concentrations, osmosis is observed. Solutions that have the same osmotic pressure are called isotonic. If two solutions have different osmotic pressure, then the solution with a high osmotic pressure is hypertonic with respect to the second, and the second is hypotonic with respect to the first. When cells are placed in an isotonic solution, they retain their size and function normally. When cells are placed in a hypotonic solution, water from a less concentrated external solution passes into the cells, which leads to their swelling, and then to the rupture of the membranes and the outflow of cellular contents. This destruction of cells is called lysis, in the case of red blood cells this process is called hemolysis. Blood with cellular contents coming out during hemolysis is called varnish blood because of its color. When cells are placed in a hypertonic solution, water leaves the cells in a more concentrated solution, and wrinkling (drying) of the cells is observed. This phenomenon is called plasmolysis. Human biological fluids (blood, lymph, tissue fluids) are aqueous solutions of low-molecular compounds - NaCI, KCl, CaCl, high-molecular compounds - proteins, polysaccharides, nucleic acids and formed elements - erythrocytes, leukocytes, platelets. Their total action determines the osmotic pressure of biological fluids. The osmotic pressure of human blood at 310°K (37°C) is 780 kPa (7,7 atm). The same pressure is created by a 0,9% NaCl aqueous solution (0,15 mol/l), which is therefore isotonic with blood (saline). However, in addition to Na and C1 ions, there are other ions in the blood, as well as IUDs and formed elements. Therefore, for medical purposes, it is more correct to use solutions containing the same components and in the same quantity as those that make up the blood. These solutions are used as blood substitutes in surgery. The human body, in addition to osmotic pressure, is characterized by constancy (homeostasis) and other physicochemical indicators of blood, such as acidity. Permissible fluctuations in the osmotic pressure of the blood are very small and even in severe pathology do not exceed several tens of kPa. In various procedures, only isotonic solutions can be injected into the blood of humans and animals in large quantities. With large blood losses (for example, after major operations, injuries), patients are injected with several liters of isotonic solution to compensate for the loss of fluid with blood. The phenomenon of osmosis is widely used in medical practice. So, in surgery, hypertonic dressings are used (gauze soaked in a hypertonic 10% NaCl solution), which are injected into purulent wounds. According to the law of osmosis, the current of the wound fluid through the gauze is directed outward, as a result of which the wound is constantly cleansed of pus, microorganisms and decay products. 20. Degree of dissociation (ionization). The strength of electrolytes Electrolytes that almost completely dissociate into ions (ionize) are called strong electrolytes, and electrolytes that are not completely ionized are called weak electrolytes. In a solution of weak electrolytes, along with ions, there are non-ionized molecules. It was by incomplete ionization that S. Arrhenius explained why the isotonic coefficient of solutions of weak electrolytes is not equal to an integer. To quantitatively characterize the completeness of dissociation, the concept of the degree of dissociation (ionization) is introduced. The degree of dissociation (ionization) of an electrolyte is the ratio of the number of molecules decomposed into ions to the total number of its molecules introduced into the solution. In other words, an is the proportion of electrolyte molecules decomposed into ions. The degree of dissociation an is expressed as a percentage or fractions of a unit: αн =Nн/ Np, where N is the number of electrolyte molecules decomposed into ions; Np the number of electrolyte molecules introduced into the solution (dissolved). So for C(CHXNUMXCOOH) = 0,1 mol/l, degree of dissociation αн = 0,013 (or 1,3%). According to the degree of dissociation, electrolytes are conventionally divided into strong (αн > 30%) and weak (αн < 3%). In the interval, electrolytes are considered to be of medium strength. Almost all salts are considered strong electrolytes. Of the most important acids and bases, H2SO4 , HCI, HBr, HI, HNO3 , NaOH, KOH, Ba(OH)2 . Weak electrolytes include most organic acids, as well as some inorganic compounds - H2S, HCN, N2WITH3 , SO3 , HCl0, N2O, N3IN3 ,Hg2CI2 ,Fe(SCN)3 . An is determined experimentally by measuring the deviation of the collative properties of electrolyte solutions from the theoretical dependences for ideal solutions. For example, the isotonic coefficient i is determined by the cryoscopic method, then the degree of dissociation is calculated. For strong electrolytes, the degree of dissociation is apparent, since they dissociate into ions almost completely. The deviation of the isotonic coefficient i from integer knowledge is explained for them not by the presence of unassociated molecules in the solution, but by other reasons. Dissociation is accompanied by the release or absorption of heat. Therefore, the degree of dissociation must depend on temperature. The influence of temperature can be estimated according to Le Chatelier's principle. If the electrolytic dissociation occurs with the absorption of heat, then with increasing temperature, an increases, if with the release of heat, an decreases. The degree of electrolytic dissociation is affected by the concentration of the solution. When the solution is diluted, the degree of dissociation increases significantly. In this regard, the indicated classification of the strength of electrolytes according to the degree of dissociation αн valid only for solutions with a concentration of about 0,1 mol/l. If we consider electrolytic dissociation as an equilibrium reversible process, then, in accordance with Le Chatelier's principle, dilution with water increases the number of distilled molecules, i.e., the degree of dissociation increases. The degree of dissociation of weak electrolytes is also affected by the addition of ions of the same name. Thus, the introduction of a weak electrolyte into the equilibrium system increases the concentration of ions, which, in accordance with the Le Chatelier principle, leads to a significant shift of the dissociation equilibrium to the left, i.e., a decrease in the degree of dissociation. Thus, the addition of ions of the same name to a weak electrolyte solution reduces the degree of its dissociation. 21. Dissociation constant. Ostwald's breeding law. Theory of solutions of strong electrolytes Quantitatively, electrolytic dissociation as an equilibrium reversible process can be characterized by a dissociation (ionization) constant determined by the law of mass action. The law of mass action, strictly speaking, applies to reversible reactions, that is, to solutions of weak electrolytes. For example, the dissociation of the KtnAnm electrolyte can be represented as an equilibrium process: Ktn Anm × nKtm+ +mAn . According to the law of mass action, the equilibrium constant is written as follows: КД = (Ktm+)n+(Ann)m + (KtnAnm) where (Ktm+) and (Ann ) - molar equilibrium concentrations of electrolyte ions; (KtnAnm) is the molar equilibrium concentration of undissociated electrolyte molecules; КД is the equilibrium constant, called the dissociation constant. This equation is valid only for dilute solutions of weak electrolytes. When applying it to concentrated solutions and to solutions of strong electrolytes, the equation must be modified. The larger the dissociation constant KД , the more the electrolyte dissociates. In contrast to the degree of dissociation KД depends only on the nature of the solvent, electrolyte and temperature, but does not depend on the concentration of the solution. Thus, both the constant and the degree of electrolytic dissociation are quantitative characteristics of dissociation. Naturally, there is a connection between them. Polybasic acids and polyacid bases dissociate in steps. For example, the dissociation of phosphoric acid occurs in three steps:  Similarly for polyacid bases (for example, Ca (OH)2) - dissociation takes place in two stages. Stepwise dissociation is characterized by the fact that the decomposition of the electrolyte at each subsequent stage occurs to a lesser extent than at the previous one. This character of the change in dissociation constants can be explained by electrostatic attraction on the basis of Coulomb's law. The ionization energy is at a minimum when an ion is detached from a neutral electrolyte molecule. The detachment of an ion at each next step of dissociation requires increasing energy, since the removal of an ion occurs from a particle whose charge becomes larger at subsequent steps. The almost complete dissociation of strong electrolytes into ions, regardless of the concentration of their solutions, is confirmed by physical and physicochemical research methods. Thus, the values of the heat of neutralization of all strong acids by strong bases in dilute solutions are almost the same. Regardless of the nature of the acid and base, the same value of AH = 56,5 kJ / mol is obtained. This fact is a clear proof of the complete dissociation of dilute solutions of acids and bases. In all cases, the common process occurring during neutralization is the combination of ions in a mol 22. Theory of acids and bases Many electrolytes, in particular hydroxides of various elements E, exhibit the properties of acids or bases. The dissociation of EON hydroxide can proceed in two types: 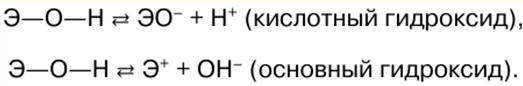 The gap can occur along both bonds of the group E-O-N. As is known, the polarity and strength of bonds depend on the difference in the electronegativity of the elements, the size and effective charge of the atoms. If the breaking energy of the O-H bond is much less than the breaking energy of the E-O bond, then the dissociation of the hydroxide proceeds according to the acid type. If, on the contrary, the energy of breaking the O-H bond is much greater than the energy of breaking the E-O bond, then the dissociation proceeds according to the main type. In hydroxides of alkali and alkaline earth metals, as well as transition metals in low oxidation states, the strength of the E-O bond is relatively low, oxygen is more strongly bonded to hydrogen, and E-O-H dissociation proceeds mainly according to the basic type, i.e., with the elimination of hydroxide dione . This is due to the fact that the ions of such elements are rather large and have a small effective charge, i.e., they have a weak polarizing ability. With an increase in the degree of oxidation, the polarizing effect of the E atom increases (an increase in the specific charge), oxygen is more firmly bound to the element E, and the dissociation of E-O-H proceeds mainly according to the acid type, i.e., the hydrogen ion is split off. The latter is connected with the redistribution of the electron density at the oxygen atom. As a result, the E-O bond becomes stronger, and the O-H bond becomes weaker. Currently, there is no unambiguous definition of the concepts of acid and base, which could equally be used to characterize acid-base interactions in any solvents. To characterize many electrolytes in aqueous solutions, it is still possible to use the concepts of acid and base given by Arrhenius: 1) an acid is an electrolyte that dissociates in solutions to form hydrogen ions H; 2) the base is an electrolyte that dissociates in solutions with the formation of OH hydroxide ions; 3) ampholyte (amphoteric hydroxide) is an electrolyte that dissociates in solution to form both hydrogen ions and hydroxide ions. Ampholytes include hydroxides of zinc, aluminum, chromium and other amphoteric elements, as well as amino acids, proteins, nucleic acids. The application of Le Chatelier's principle to the chain of acid-base equilibria shows that with an increase in the concentration of OH hydroxide ions in the system, the probability of acid-type dissociation increases. An increase in the concentration of hydrogen ions H+ in the system leads to predominant dissociation according to the main type. This means that in an acidic environment, the ampholite exhibits a basic character, and in an alkaline environment, it exhibits an acidic character. For example, zinc hydroxide behaves like a base when interacting with acids: Zn (OH)2 + 2HCI - ZnCI2 + 2Н2Oh, and when interacting with alkalis - as an acid: Zn(OH)2+ 2NaOH → Na2[Zn(OH)4]. 23. Blood buffer systems. Blood plasma Buffer systems are of great importance in maintaining the acid-base balance of organisms. Intracellular and extracellular fluids of all living organisms are characterized by a constant pH value, which is maintained with the help of buffer systems. The pH value of most intracellular fluids is in the range from 6,8 to 7,8. The acid-base balance of KO balance in human blood is provided by hydrogen-carbonate, phosphate and protein buffer systems. The normal pH value of blood plasma is 7,40 ± 0 05. This corresponds to the range of active acidity values from 3,7 to 4,0x108 mol/l. Since there are various electrolytes in the blood (HC03 , H2CO3 H2Ro4 , NRO42 ), proteins, amino acids, which means that they dissociate to such an extent that the activity of a (H +) is in the indicated range. Due to the fact that the content of inorganic and organic substances in plasma and blood cells is not the same, it is advisable to consider these blood components separately. Blood plasma HCO buffer system3 / N2WITH3 is made up of carbonic acid2WITH3 and conjugate base HCO3 . It is the most important buffer system in the blood. One of the components is carbonic acid H2WITH3 - formed by the interaction of CO dissolved in plasma2 with water: WITH2(r) + N2He N2WITH3. where CO2(r) - concentration of dissolved CO2 . The equilibrium constant of this reaction is: K = [N2WITH3] / [CO2] Between CO2 in the alveoli and hydrogen-carbonate buffer in the blood plasma flowing through the capillaries of the lungs, a chain of equilibria is established. The hydrogen carbonate buffer system acts as an effective physiological buffer near pH 7,4. Upon entry into the blood of acids - donors of H+ the equilibrium in the chain according to the Le Chatelier principle shifts to the left as a result of the fact that HCO ions3 bind H ions into H molecules2WITH3 . At the same time, the concentration of H2WITH3 increases, and the concentration of HCO ions3 goes down. Increasing the concentration of H2WITH3 leads to a shift of equilibrium to the left, (Le Chatelier's principle). It causes decay2WITH3 and an increase in CO concentration2 dissolved in plasma. As a result, the equilibrium shifts to the left and the pressure of CO increases.2 in the lungs. Excess CO2 excreted from the body. As a result, the hydrogen-carbonate system of the blood quickly comes into equilibrium with CO2 in the alveoli and effectively maintains the constancy of the pH of the blood plasma. 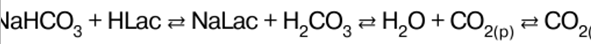 Thus, a normal blood pH value is maintained with a slightly pronounced pH shift due to acidosis. In enclosed spaces, they often experience suffocation (lack of oxygen), increased breathing. However, suffocation is associated not so much with a lack of oxygen, but with an excess of CO2. Excess CO2 in the atmosphere, according to Henry's law, leads to additional dissolution of CO2 in blood. And this leads to a decrease in blood pH, i.e. to acidosis. The hydrogen-carbonate buffer system most quickly responds to changes in blood pH. Its acid buffer capacity is Vк \u40d XNUMX mmol / l of blood plasma, and the buffer capacity for alkali is much less and is approximately equal to Vщ = 1-2 mmol/l of blood plasma. 24. Neutralization reactions Neutralization reactions are called exchange reactions of the interaction of acids and bases, as a result of which salt and water are formed. Consider different types of neutralization reactions. 1. Neutralization of a strong base with a strong acid: KOH + HNO3 -KNO3 + N2O. The molecular ionic equation for such a reaction H+ + OH → N2O and the negative value of the Gibbs energy ΔG° show that the equilibrium is practically shifted towards the formation of water. A common case of a neutralization reaction is the interaction of acids and bases that differ in strength (the degree of dissociation). These reactions do not go to completion due to the reverse reaction of salt hydrolysis. 2. Neutralization of a weak acid with a strong base:  or in molecular form:  In this case, the neutralization reaction is reversible. The reaction of neutralization of a weak base with a strong acid is also reversible:  or in molecular form: 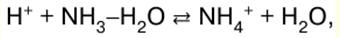 and also - a weak base with a weak acid: 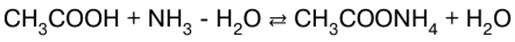 or in molecular form:  In these systems, the equilibrium is strongly shifted to the right, since water is a much weaker electrolyte than hydrocyanic acid, ammonia and acetic acid. Neutralization reactions form the basis of the neutralization method. This method is used in clinical laboratories to determine the acidity of gastric juice, the buffer capacity of blood plasma. In pharmacology, it is used for the quantitative analysis of inorganic acids (hydrochloric, sulfuric, boric) and organic acids (acetic, benzoic, tartaric, citric, salicylic). In biopharmaceutical studies, the pKa of acids and pKa of bases are determined by neutralization, since the value of these values can predict the ability of drugs to pass through biological membranes. Acid-base titration is used to determine pKа amino acids and pKа dissociating groups in proteins. Protein titration curves obtained at two different temperatures can be used to determine the number of carboxyl, imidazole, and other groups. Titration of amino acids and proteins makes it possible to determine their isoelectric points. Hydrolysis is the decomposition of a substance by water. Chemical compounds of various classes can undergo hydrolysis: proteins, fats, carbohydrates, esters, salts, etc. In inorganic chemistry, they are most often encountered with the hydrolysis of salts. 25. Salt hydrolysis Salt hydrolysis - this is the interaction of salt with water molecules, leading to the formation of low-dissociation compounds. The process of hydrolysis consists in the transition of a proton from a water molecule to a given ion (CO32 + HOH * HCO3+ OH ) or from a given ion, including from a hydrated metal cation, to a water molecule. Depending on the nature of the salt, water acts either as an acid or as a base, and the salt is, respectively, the conjugate base or the conjugate acid. Four variants of hydrolysis are possible depending on the type of salt. 1. Salts formed by a strong acid and a weak base:  2. Salts formed by a strong base and a weak acid: CH3COONa + HOH → CH3COOH + NaOH. 3. Salts formed by a weak acid and a weak base. Ammonium cyanide is hydrolyzed by the reaction: 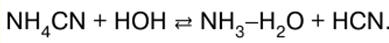 4. Salts formed by a strong acid and a strong base. NaCl, KNO3 do not undergo hydrolysis. The mechanism of salt hydrolysis consists in the polarization interaction of salt ions with their hydration shell. The stronger this interaction, the more intense the hydrolysis. All the considered cases of hydrolysis concerned salts formed by one-acid bases and one-basic acids. Salts of polybasic acids and polyacid bases are hydrolyzed stepwise, forming acidic and basic salts. Quantitatively, hydrolysis as a CO equilibrium is characterized by the degree of hydrolysis and the hydrolysis constant. The degree of hydrolysis is measured by the ratio of the amount of hydrolyzed substance to the total amount of solute. The degree of hydrolysis depends on the nature of the salt, its concentration and temperature. According to the law of mass action, the degree of hydrolysis increases with dilution of the solution. So, at a concentration Na2CO3 0,001 mol/l, the degree of hydrolysis is 34%. In the general case, the following regularities are true. 1. Salt hydrolysis should increase with increasing temperature and dilution of the solution. 2. In reversible hydrolysis, in accordance with the Le Chatelier principle, the process should be suppressed by acidification (if this salt is formed by a strong acid and a weak base, H ions accumulate) or by alkalization (if the salt is formed by a weak acid and a strong base, OH ions accumulate). 3. The hydrolysis of salts, which results in the formation of poorly soluble or gaseous products that are removed from the reaction sphere (the principle of equilibrium shift), is irreversible. For example, hydrolysis of Pb(SO4)2 proceeds entirely due to the formation of a precipitate of PbO2: Pb(SO4)2 + 2Н2O→PbO2 + 2H2SO4. Hydrolysis is characteristic of many classes of inorganic and organic compounds. Hydrolysis of inorganic compounds is important for assessing their toxicity. Hydrolysis of organic compounds is used to obtain valuable products from wood, fats, esters, and other things, but hydrolysis plays a particularly important role in the life of living organisms. 26. Precipitation and dissolution reaction The exchange reactions occurring in the electrolyte solution include the reactions of precipitation and dissolution. Precipitation reactions are accompanied by precipitation. Reactions accompanied by the dissolution of precipitates are called dissolution reactions. Systems consisting of a deposit of a sparingly soluble electrolyte and a saturated solution above it are widely used. In such systems, a dynamic equilibrium is established between the saturated solution and the precipitate. Due to the low solubility, the concentration of the sparingly soluble electrolyte in the solution is very low; therefore, it can be considered that it is completely dissociated in the solution. In other words, dynamic equilibrium in a saturated solution is established between the solid phase of the substance and the ions that have passed into the solution. For example, in a saturated solution of AgCl, the following equilibrium takes place: AgCl(T) → Ag+(P) + Cl(R). The concentration of the solid phase AgCl as a constant value is excluded from the expression for the equilibrium constant. As a result, the equilibrium constant is determined only by the product of the ion concentrations in the solution and is called the constant or the solubility product. In the general case, for the Ktn Anm electrolyte, the solubility constant is determined by the stoichiometric product of the ion concentrations: Кetc.= [Ktm+ ]n[Ann]m This value characterizes the solubility of the electrolyte at a constant temperature in the absence of foreign substances. K constancyetc. does not mean the constancy of the concentrations of individual ions in solution. Thus, it is possible to increase the concentration of Ag ions in a saturated solution of AgCl by adding, for example, AgNO3 , while the equilibrium according to the Le Chatelier principle will shift to the left, which will lead to an increase in the rate of ion deposition. After some time, the dissolution rates of AgCl and the precipitation of Ag and Cl ions will become equal. The newly established equilibrium will, as before, be characterized by the value Ketc.(AgCl), but the equilibrium concentrations of Ag and Cl ions will change. Thus, based on Ketc. it is possible to predict the formation and dissolution of electrolyte precipitates based on two rules. 1. An electrolyte precipitates when the stoichiometric product of the concentrations of its ions in solution is greater than the solubility constant. 2. The electrolyte precipitate dissolves when the stoichiometric product of the concentrations of its constituent ions in solution becomes less than the solubility constant. Precipitation reactions form the basis of the precipitation method used in the quantitative analysis of pharmaceuticals. The precipitation method is used in the clinical analysis of chlorides in urine, gastric juice, blood, in sanitary and hygienic practice - in the analysis of drinking water. Scientists believe that the different solubility of natural compounds of elements in water had a great influence on their content in living organisms. There is a close relationship between the solubility of compounds in water and the toxic effect of ions of a number of elements. For example, the introduction of Al3 + into the body due to the formation of poorly soluble aluminum phosphate AlPO4 27. Redox reactions One of the basic concepts of inorganic chemistry is the concept of oxidation state (CO). The oxidation state of an element in a compound is the formal charge of the element's atom, calculated from the assumption that valence electrons pass to atoms with a higher relative electronegativity (REO) and all bonds in the compound molecule are ionic. The oxidation state of the element E is indicated at the top above the element symbol with a "+" or "-" sign before the number. The degree of oxidation of ions that actually exist in a solution or crystals coincides with their charge number and is denoted similarly with the sign "+" or "-" after the number, for example, Cl,Ca2+. The Stock method is also used to indicate the degree of oxidation in Roman numerals after the symbol of the element: Mn (VII), Fe (III). The question of the sign of the oxidation state of atoms in a molecule is solved on the basis of a comparison of the electronegativities of the interconnected atoms that form the molecule. In this case, an atom with a lower electronegativity has a positive oxidation state, and with a higher electronegativity - a negative one. It should be noted that the oxidation state cannot be identified with the valency of the element. Valence, defined as the number of chemical bonds by which a given atom is connected to other atoms, cannot be zero and does not have a "+" or "-" sign. The oxidation state can have both positive and negative values, as well as zero and even fractional values. For example, in a CO molecule2 the oxidation state of C is +4, and in the CH molecule4 the oxidation state of C is 4. The valence of carbon4 a in both compounds is IV. Despite the above disadvantages, the use of the concept of the degree of oxidation is convenient in the classification of chemical compounds and the formulation of equations for redox reactions. When an element is oxidized, the oxidation state increases, in other words, the reducing agent during the reaction increases the oxidation state. On the contrary, when the element is reduced, the oxidation state decreases, i.e., during the reaction, the oxidizing agent reduces the oxidation state. Thus, it is possible to give the following formulation of redox reactions: redox reactions are reactions that occur with a change in the oxidation state of the atoms of the elements that make up the reacting substances. 28. Oxidizing and reducing agents To predict the products and the direction of redox reactions, it is useful to remember that typical oxidizing agents are simple substances whose atoms have a large EER> 3,0 (elements of VIA and VIIA groups). Of these, the most powerful oxidizing agents are fluorine (OEO = 4,0), oxygen (OEO = 3,0), chlorine (OEO = 3,5). Important oxidants include PbO2 , KNO4 , Ca(SO4)2 , K2Cr2O7 , HClO, HClO3, KSIO4, NaBio3, H2SO4(end),HNO3(end) Na2O2 , (NH4)2S2O8 , KSIO3 , H2O2 and other substances that contain higher or higher CO atoms. Typical reducing agents include simple substances whose atoms have a small EER < 1,5 (metals of IA and IIA groups and some other metals). Important reducing agents include H2S, N.H.3, HI, KI, SnCl2 , FeSO4 ,C,H2 ,CO,H2SO3 , cr2(SO4)3 , CuCl, Na2S2O3 and other substances that contain low CO atoms. Substances containing atoms in the maximum and minimum oxidation states, respectively, can only be oxidizing agents, for example, K2sg2O7 , KMPO4 , PbO2 , HClO4 or only reducing agents, such as NH3 , H2S, HI. Substances containing atoms in intermediate oxidation states are capable of both raising and lowering the oxidation state, that is, they can be both reducing agents (under the action of a more active oxidizing agent than them) and oxidizing agents (under the action of a more active than they , reducing agent). Such substances exhibit redox duality. When compiling the equations of redox reactions, two methods can be used: the electron balance method and the ion-electron method (half-reaction method). A more correct idea of redox processes in solutions is given by the ion-electron method. With the help of this method, changes are predicted that ions and molecules actually existing in a solution undergo. In addition to predicting reaction products, ionic half-reaction equations are necessary for understanding redox processes occurring during electrolysis and in galvanic cells. This method reflects the environment's role as a participant in the process. And finally, when using this method, it is not necessary to know in advance all the substances formed, since many of them are obtained by compiling the equation of redox reactions. It should be borne in mind that although half-reactions reflect the real processes occurring during redox reactions, they cannot be identified with the real stages (mechanism) of redox reactions. Many factors influence the nature and direction of redox reactions: the nature of the reactants, the reaction of the medium, concentration, temperature, and catalysts. It should be borne in mind that a negative value does not always lead to an unambiguous decision about the actual course of the reaction in a given direction, since it is additionally necessary to take into account the kinetic factor. 29. Biological significance of redox processes Redox reactions are chemical processes accompanied by the transfer of electrons from one molecule or ion to another. In redox reactions, two interrelated processes occur: oxidation and reduction. Oxidation is the process of losing electrons. Recovery is the process of adding electrons. Substances whose atoms or ions donate electrons are called reducing agents. Substances whose atoms or ions add electrons (or pull a common pair of electrons to themselves) are called oxidizing agents. In the reaction of zinc with CuSO4 Cu2 + add electrons: Si2+ + 2e־ - Si0 . Zinc atoms donate electrons: Zn0 -Zn2 + 2e־. Accordingly, CuSO4 - oxidizing agent, Zn - reducing agent. Important processes in animal organisms are the reactions of enzymatic oxidation of substrate substances: carbohydrates, fats, amino acids. As a result of these processes, organisms receive a large amount of energy. Approximately 90% of the total energy requirement of an adult male is covered by the energy produced in the tissues during the oxidation of carbohydrates and fats. The rest of the energy - ~10% is provided by the oxidative breakdown of amino acids. Biological oxidation proceeds through complex mechanisms with the participation of a large number of enzymes. In mitochondria, oxidation occurs as a result of electron transfer from organic substrates. As electron carriers, the respiratory chain of mitochondria includes various proteins containing various functional groups that are designed to carry electrons. As they move along the chain from one intermediate to another, the electrons lose their free energy. For every pair of electrons transferred through the respiratory chain to oxygen, 3 ATP molecules are synthesized. The free energy released during the transfer of 2 electrons to oxygen is 220 kJ/mol. The synthesis of 1 ATP molecule under standard conditions consumes 30,5 kJ. From this it is clear that a fairly significant part of the free energy released during the transfer of one pair of electrons is stored in ATP molecules. From these data, the role of multistage electron transfer from the initial reducing agent to oxygen also becomes clear. The large energy (220 kJ) released during the transfer of one pair of electrons to oxygen is divided into a number of portions corresponding to individual stages of oxidation. At three such stages, the amount of energy released approximately corresponds to the energy required for the synthesis of 1 ATP molecule. Redox reactions underlie the methods of oxidimetry, which are used in clinical analysis to determine Ca ions, uric acid, catalase and peroxidase enzymes, sugar in the blood, and in sanitary and hygienic analysis to determine the oxidizability of water, the content of active chlorine in bleach, residual chlorine in household and drinking water. water 30. Chemical bond and its experimental characteristics The development of a modern model of the atom and the prediction of the properties of individual atoms on its basis is a very important achievement of quantum mechanics. However, isolated atoms are rarely found under terrestrial conditions. The bodies of inanimate and living nature around us consist of a variety of molecules. A. M. Butlerov (1828-1886) created the theory of the chemical structure of organic substances (1861). Since that time, the concepts of "valence" and "chemical bond" gradually began to enter into chemistry. Valency is the ability of an atom to attach a certain number of other atoms to form a molecule. Valency is indicated by dashes next to the element symbol. Hydrogen (H) is monovalent, oxygen (0= ) is divalent. The number of valence lines determines the number of chemical bonds that a given atom can form with other atoms. A chemical bond is a set of interactions between electrons and nuclei, leading to the combination of atoms into a molecule. The properties of a chemical bond are studied by various methods. With the help of chemical methods, the number of bonds of atoms (valency) and their reactivity are determined. Using physical methods, the length, strength, orientation and polarity of chemical bonds are determined. Chemical bond length rс called the value measured by the distance between the nuclei of the bound atoms. As a unit of chemical bond length gс convenient to use picometer (pm): 1 pm = 1012 м. Chemical bond strength Eс - value measured by enthalpy ΔЕс connection formation. As a unit of chemical bond strength Eс kJ/mol is used. Chemical bond orientation aс - the value measured by the angle between the directions of the bonds of a given atom with neighboring atoms of the molecule. The angle ac is called the valence angle. Bond angle unit aс - degree. Chemical bond polarity μс - the value measured by the electric moment of this connection. The electric moment for two electric charges +q and ־q, equal in absolute value and opposite in sign, is equal to μ = qr, where r is the distance between the charges. These two charges form an electric dipole. A chemical bond polarizes when 2 atoms with different electronegativity (EEO) bond. As a result, an excess negative charge δ arises on an atom with a large EER, and an excess positive charge +δ arises on another atom with a smaller EER. The polarity of the bond is calculated by the formula: μс = δrс. As a unit for measuring the polarity of a chemical bond, it is convenient to use the off-system Debye unit (D) - 1 D = 3,3 x 1030 C/m. The polarity of the O-H bond in the water molecule is μhe = 1,5D. The study of the chemical bond showed that in most cases the length, strength, orientation, polarity of the same chemical bond in different compounds have approximately the same values. Hence it follows that the interactions leading to the formation of a given bond between atoms are of the same nature in different molecules. Quantum mechanical theories of chemical bonding provide an explanation for this fact. 31. Hydrogen bond. Intermolecular and intramolecular hydrogen bonding Chemical bonds in molecules are usually very strong, their energy is in the range of 100-150 kJ/mol. In addition, there are so-called hydrogen bonds, the strength of which is 10-40 kJ/mol. The length of these bonds is 270-230 pm, respectively. Hydrogen bond between atoms EА and EВ called the interaction carried out by a hydrogen atom connected to EА or EВ chemical bond. The image of a hydrogen bond in the general case has the form: ЭА-N...Eв.. Obviously, the hydrogen bond is three-center, since 3 atoms take part in its formation. For the occurrence of such a bond, it is necessary that the atoms EА and EВ have high electronegativity. These are the atoms of the most negative elements: nitrogen (REO = 3,0), oxygen (REO = 3,5), fluorine (REO = 4,0) and chlorine (REO = 3,0). A hydrogen bond is formed as a result of a combination of ls-AO hydrogen and two 2pAO atoms EА and EВ; 2rorbitals are oriented along one straight line. Therefore, the hydrogen bond is linear. The hydrogen bond is called: 1) intramolecular, if the atoms EА and EВ , connected by this bond, belong to the same molecule; 2) intermolecular, if atoms EА and EВ are in different molecules. Intramolecular hydrogen bonds play an important biological role, since they determine, for example, the helical structure of polymeric protein molecules. In proteins, these are N-H ... O bonds between amino acid residues. Equally important are intermolecular hydrogen bonds. With their help, chains of nucleic acids are connected, forming a double helix. There are two types of bonds between nucleic bases - NHN and N-H-O. The average kinetic energy of the thermal motion of molecules is of the order of 3/2RT. At a human body temperature of 37°C (310°K), this is about 4 kJ/mol. The strength of hydrogen bonds is in the range of 10-40 kJ / mol, so they are strong enough to withstand constant impacts of surrounding molecules and ensure the constancy of the shape of polymeric biological structures. At the same time, upon impact of active molecules, hydrogen bonds are periodically broken, then restored again, ensuring the flow of various life processes. The considered examples clearly illustrate a wider range of applications of the MO LCAO method than the VS method. Nevertheless, the VS method can be successfully used to predict the properties and structure of many substances, including complex compounds. 32. Macro and microelements in the environment and in the human body There are various classifications of chemical elements contained in the human body. So, V. I. Vernadsky, depending on the average content (mass fraction w, %) in living organisms, divided the elements according to the ten-day system. According to this classification, the elements contained in living organisms are divided into three groups: macro, micro and ultramicroelements. Macronutrients These are elements whose content in the body is higher than 102%. These include oxygen, carbon, hydrogen, nitrogen, phosphorus, sulfur, calcium, magnesium, sodium, and chlorine. Trace Elements These are elements whose content in the body is in the range of 103 to 105%. These include iodine, copper, arsenic, fluorine, bromine, strontium, barium, cobalt. Ultramicroelements These are elements whose content in the body is below 105%. These include mercury, gold, uranium, thorium, radium, etc. Currently, ultramicroelements are combined with microelements into one group. This classification reflects only the content of elements in living organisms, but does not indicate the biological role and physiological significance of this or that element. V. V. Kovalsky, based on their importance for life, divided the chemical elements into three groups. Vital (irreplaceable) elements They are constantly contained in the human body, are part of enzymes, hormones and vitamins: H, O, Ca, N, K, P, Na, S, Mg, d, C, I, Mn, Cu, Co, Fe, Zn, Mo, V. Their deficiency leads to disruption of normal human life. impurity elements These elements are constantly found in the body of animals and humans: Ga, Sb, Sr, Br, F, B, Be, Li, Si, Sn, Cs, Al, Ba, Ge, As, Rb, Pb, Ra, Bi, Cd, Cr, Ni, Ti, Ag, Th, Hg, U, Se. Their biological role is little understood or unknown. impurity elements Sc, Tl, In, La, Pr, Sm, W, Re, Tb, etc. Found in humans and animals. Data on the number and biological role have not yet been clarified. The elements necessary for the construction and vital activity of various cells and organisms are called biogenic elements. It is still impossible to accurately list all biogenic elements due to the difficulty of determining very low concentrations of trace elements and establishing their biological functions. For 24 elements, the biogenicity was reliably established. These are elements of the first and some elements of the second group (according to Kowalski). 33. Topography of the most important biogenic elements in the human body Human organs differently concentrate various chemical elements in themselves, i.e. micro and macro elements are unevenly distributed between different organs and tissues. Most trace elements accumulate in the liver, bone and muscle tissues. These tissues are the main depots (stores) for many trace elements. Elements may show a specific affinity for certain organs and be contained in them in high concentrations. It is well known that zinc is concentrated in the pancreas, iodine - in the thyroid gland, fluorine - in tooth enamel, aluminum, arsenic, vanadium accumulate in hair and nails, cadmium, mercury, molybdenum - in the kidneys, tin - in the intestinal tissues, strontium - in prostate gland, bone tissue, barium - in the pigmented retina of the eye, bromine, manganese, chromium - in the pituitary gland, etc. In organisms, trace elements can be in a bound state and in the form of free ionic forms. It is known that silicon, aluminum, copper and titanium in brain tissues are in the form of complexes with proteins, while manganese is in ionic form. Hydrogen and oxygen are macronutrients. They are part of the water, which in the body of an adult on average contains about 65%. Water is unevenly distributed over human organs, tissues and biological fluids. So, in gastric juice, saliva, blood plasma, lymph, water is from 89,5 to 90%, in urine, gray matter of the brain, kidneys - 80%, in the white matter of the brain, liver, skin, spinal cord, muscles, lungs, heart - 70-80%. Least of all - 40% of water - is contained in the skeleton. Macronutrients - carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus - are part of proteins, nucleic acids and other biologically active compounds of the body. The carbon content in proteins is 51-55%, oxygen - 22-24%, nitrogen - 15-18%, hydrogen - 6,5-7%, sulfur - 0,3-2,5%, phosphorus - about 0,5 %. Carbon, hydrogen and oxygen are also part of carbohydrates, the content of which in animal tissues is low - about 2%. These elements are part of lipids (fats). In addition, the composition of phospholipids includes phosphorus in the form of phosphate groups. To the greatest extent, lipids are concentrated in the brain (12%), then in the liver (5%), milk (2-3%) and blood serum (0,6%). However, the main part of phosphorus (600 g) is found in bone tissue. This is 85% of the mass of all phosphorus in the human body. Phosphorus is also concentrated in the hard tissues of the teeth, in which it is included together with calcium, chlorine, fluorine in the form of hydroxyl, chlorine, fluorapatites of the general formula Ca5 (PO4)3X, where X = OH, CI, F, respectively. Calcium is predominantly concentrated in bone, as well as in dental tissues. Sodium and chlorine are mainly found in extracellular fluids, while potassium and magnesium are found in intracellular fluids. In the form of fluorides, sodium and potassium are part of the bone and dental tissues. Magnesium as Mg Phosphate3 (PO4)2 contained in the hard tissues of the tooth. Hormones are involved in maintaining a certain content of macro- and microelements in the body. 34. The biological role of chemical elements in the body The biological role of chemical elements in the human body is extremely diverse. The main function of macronutrients is to build tissues, maintain a constant osmotic pressure, ionic and acid-base composition. Trace elements, being part of enzymes, hormones, vitamins, biologically active substances as complexing agents or activators, are involved in metabolism, reproduction processes, tissue respiration, and neutralization of toxic substances. Trace elements actively influence the processes of hematopoiesis, oxidation, reduction, permeability of blood vessels and tissues. Macro and microelements - calcium, phosphorus, fluorine, iodine, aluminum, silicon - determine the formation of bone and dental tissues. Many diseases associated with a deficiency or excessive accumulation of various trace elements have been identified. Fluorine deficiency causes dental caries, iodine deficiency - endemic goiter, excess molybdenum - endemic gout. Such patterns are connected with the fact that the balance of optimal concentrations of biogenic elements is maintained in the human body - chemical homeostasis. Violation of this balance due to a lack or excess of the element can lead to various diseases. In addition to the six main macronutrients - organogens (carbon, hydrogen, nitrogen, oxygen, sulfur and phosphorus), which make up carbohydrates, fats, proteins and nucleic acids, inorganic macronutrients - calcium, chlorine, magnesium, potassium, sodium - and trace elements - copper, fluorine, iodine, iron, molybdenum, zinc, and also, possibly (it has been proven for animals), - selenium, arsenic, chromium, nickel, silicon, tin, vanadium. Analysis of the content and ratio of microelements in the human body is also used in forensic medical examination. For example, in the case of alcohol poisoning, under the influence of ethyl alcohol, the calcium content in the liver increases, and sodium and potassium become less. At the same time, in the heart and kidneys, on the contrary, the calcium content decreases. The lack of elements such as iron, copper, fluorine, zinc, iodine, calcium, phosphorus, magnesium and some others in the diet leads to serious consequences for human health. However, it must be remembered that not only a deficiency, but also an excess of biogenic elements is harmful to the body, since this disrupts chemical homeostasis. Mineral components, which are vital in negligible amounts, become toxic at higher concentrations. A number of elements (silver, mercury, lead, cadmium, etc.) are considered toxic, since their entry into the body, even in trace amounts, leads to severe pathological phenomena. Various elements and their compounds are widely used as medicines. Thus, the study of the biological role of chemical elements, the elucidation of the relationship between the exchange of these elements and other biologically active substances (enzymes, hormones, vitamins) contributes to the creation of new drugs and the development of optimal dosage regimens for both therapeutic and prophylactic purposes. 35. S-elements and their compounds Water is one of the most important and widespread hydrogen compounds on Earth. Water space occupies almost 75% of the surface of the globe. The body of an adult contains on average 65-67% of water, the fetus (4 months) - 94%, in newborns - 74%. All chemical reactions in the body occur only in the aquatic environment. Life without water is impossible. Distilled water is a pharmacopoeial preparation. In medical practice, another hydrogen compound is used - hydrogen peroxide H2 02. This compound is an important by-product of metabolism. Hydrogen peroxide is a colorless, transparent liquid. Causes burning on contact with skin and mucous membranes. Molecule H2О2 polar. The presence of lone pairs of electrons at oxygen atoms makes it possible to form donor-acceptor bonds of hydrogen peroxide with ligands - electron acceptors. The oxidation state of oxygen in H2О2 is equal to 1, i.e. it has an intermediate value between the oxidation state of oxygen in water (2) and in elemental oxygen O2 . It follows from this that hydrogen peroxide can exhibit both the properties of an oxidizing agent and the properties of a reducing agent (redox duality). However, judging by the standard redox half-reaction potentials, oxidizing properties are more characteristic of hydrogen peroxide. Pure hydrogen peroxide is thermodynamically unstable and, on standing, decomposes explosively into water and oxygen, releasing a large amount of heat. Aqueous solutions of hydrogen peroxide are more stable; in a cool place they can be stored for a long time. Hydrogen peroxide is usually sold in the form of a 30% aqueous solution - perhydrol. The co-process of decomposition of hydrogen peroxide is significantly accelerated in the presence of salts of heavy metals. The metal ion-catalyzed decomposition of hydrogen peroxide can lead to the formation of radicals, the most important of which are hydroxide HO and hydroperoxide. The toxicity is due to the fact that2О2 and O2 interact with the lipid layer of cell membranes and damage them. In medical practice, hydrogen peroxide is mainly used as an external bactericidal agent. Action H2О2 based on the oxidizing power of hydrogen peroxide and the harmlessness of its reduction product - water. When treating wounds, the released oxygen plays a dual role: 1) has an antimicrobial, deodorizing and depigmenting effect, killing microbial bodies; 2) forms foam, contributing to the transition of tissue decay particles to a suspended state and cleansing of wounds. As a pharmacopoeial preparation, a 3% aqueous solution of hydrogen peroxide is used, a 6% solution of hydrogen peroxide is used to bleach hair. In the form of a 30% solution, hydrogen peroxide is used in the treatment of the warty form of lichen planus and to remove youthful warts. 36. The biological role of s-elements of the IA group (lithium, rubidium, cesium, francium) According to the content in the human body, sodium (0,08%) and potassium (0,23%) are macronutrients, and the rest of the alkali metals are lithium (104%), rubidium (105%), cesium (104%) - to trace elements. Lithium The content of lithium in the human body is about 70 mg (10 mmol) - 104%. Lithium compounds in higher animals are concentrated in the liver, kidneys, spleen, lungs, blood, and milk. The maximum amount of lithium is found in human muscles. The biological role of lithium as a trace element has not yet been fully elucidated. It has been proven that at the level of cell membranes, Li ions (at a sufficient concentration) compete with sodium ions when penetrating into cells. Obviously, the replacement of Na ions in cells by Li ions is associated with a greater covalence of lithium compounds, as a result of which they dissolve better in phospholipids. It has been established that some lithium compounds have a positive effect on patients with manic depression. Absorbed from the gastrointestinal tract, Li ions accumulate in the blood. When the concentration of Li ions reaches 0,6 mmol / l and above, there is a decrease in emotional tension and a weakening of manic excitement. At the same time, the content of Li ions in the blood plasma must be strictly controlled. In cases where the concentration of Li ions exceeds 1,6 mmol/l, negative phenomena are possible. rubidium and cesium According to the content of rubidium in the human body (105%) and cesium (104%) belong to trace elements. They are constantly contained in the body, but their biological role has not yet been elucidated. Being a complete analogue of potassium, rubidium also accumulates in the intracellular fluid and can replace an equivalent amount of potassium in various processes. Radioactive isotopes 13rCs and 87Rb is used in radiotherapy of malignant tumors, as well as in the study of potassium metabolism. Due to their rapid breakdown, they can even be introduced into the body without fear of long-term harmful effects. France This is a radioactive chemical element obtained by artificial means. There is evidence that francium can selectively accumulate in tumors at the earliest stages of their development. These observations may be useful in the diagnosis of cancer. Thus, among the elements of the IA group, Li, Rb, Cs are physiologically active, while Na and K are vital. The proximity of the physicochemical properties of Li and Na, due to the similarity of the electronic structure of their atoms, is also manifested in the biological action of cations (accumulation in the extracellular fluid, interchangeability). A similar nature of the biological action of cations of elements of large periods - K+, Rb+, Cs+ (accumulation in the intracellular fluid, interchangeability) is also due to the similarity of their electronic structure and physicochemical properties. This is the basis for the use of sodium and potassium preparations in case of poisoning with lithium and rubidium salts. 37. The biological role of s-elements of the IA group (sodium, potassium) The sodium content in a human body weighing 70 kg is about 60 g (2610 mmol) - 0,08%. Of this amount, 44% of sodium is in the extracellular fluid and 9% in the intracellular fluid. The rest of the sodium is in the bone tissue, which is the place of deposition of the Na ion in the body. About 40% of the sodium contained in the bone tissue is involved in metabolic processes, and due to this, the skeleton is either a donor or an acceptor of Na ions, which helps to maintain a constant concentration of Na ions in the extracellular fluid. Sodium is the main extracellular ion. In the human body, sodium is in the form of its soluble salts, mainly chloride, phosphate and hydrogen carbonate. Sodium is distributed throughout the body: in blood serum, cerebrospinal fluid, eye fluid, digestive juices, bile, kidneys, skin, bone tissue, lungs, and brain. Na ions play an important role in ensuring the constancy of the internal environment of the human body, are involved in maintaining a constant osmotic pressure of the biofluid (osmotic homeostasis). Na ions are involved in the regulation of water metabolism and affect the functioning of enzymes. Together with K, Mg, Ca, Cl ions, the Na ion is involved in the transmission of nerve impulses and maintains the normal excitability of muscle cells. When the sodium content in the body changes, dysfunctions of the nervous, cardiovascular and other systems, smooth and skeletal muscles occur. Sodium chloride NaCl is the main source of hydrochloric acid for gastric juice. Sodium enters the human body mainly in the form of table salt. The body's true daily requirement for sodium is 1 g, although the average intake of this element reaches 4-7 g. Continuous excess intake of NaCl contributes to hypertension. Under the influence of alkalis on microbial cells, the precipitation of cellular proteins occurs and, as a result, the death of microorganisms. Sodium sulfate (Glauber's salt) Na2SO4 × 10H2O is used as a laxative. Sodium tetraborate Na2B4О7 × 10H2O is used externally as an antiseptic for rinsing, douching, lubricating. Sodium hydroxide in the form of a 10% solution is part of the silin used in orthopedic practice for casting refractory models in the manufacture of one-piece cast prostheses from a cobalt-chromium alloy. The content of potassium in a human body weighing 70 kg is approximately 160 g (4090 mmol) - 0,23%. Potassium is the main intracellular cation, accounting for 2/3 of the total number of active cellular cations. Of the total amount of potassium contained in the body, 98% is located inside the cells and only about 2% is in the extracellular fluid. Potassium is distributed throughout the body. Its topography: liver, kidneys, heart, bone tissue, muscles, blood, brain, etc. K ions play an important role in physiological processes - muscle contraction, normal functioning of the heart, conduction of nerve impulses, metabolic reactions. K ions are important activators of enzymes located inside the cell. 38. Biological role of s-elements of the IIA-group. Their use in medicine (beryllium, magnesium, calcium) Beryllium is found in plants as well as in animal organisms. The content of beryllium in living organisms is 107%, i.e. it is an impurity ultramicroelement. The biological role of beryllium has not been studied enough. Beryllium compounds are toxic and cause a number of diseases (beryllium rickets, berylliosis, etc.). Volatile compounds of beryllium are especially toxic. The negative effect of Be2 + on physiological processes is explained by its chemical properties. Magnesium is formally a macronutrient. Its total content in the body is 0,027% (about 20 g). The topography of magnesium in the human body is as follows: magnesium is concentrated to the greatest extent in dentin and tooth enamel, bone tissue. It also accumulates in the pancreas, skeletal muscles, kidneys, brain, liver and heart. In an adult, the daily requirement for magnesium is about 0,7 g. The Mg ion, like the K ion, is an intracellular cation. In biological fluids and tissues of the body, magnesium is found both in the form of an aqua ion and in a protein-bound state in an amount of < 102%, i.e., in essence, it is a microelement. The concentration of Mg ions inside cells is approximately 2,5-3 times higher than in extracellular fluids. Magnesium ions play an important biological role in the human body. Due to the smaller ion radius and the higher ionization energy of Mg2+ forms stronger bonds than the Ca ion, and therefore is a more active catalyst for enzymatic processes. Being part of various enzymatic systems, the Mg ion is their indispensable component and activator (enzymes such as carboxypeptidase, cholinesterase, and some others are specific for the Mg ion). Hydrolysis of ATP, associated with a number of enzymatic reactions, as a result of which hydrophosphation HPO2 is formed and a large amount of energy is released, takes place with an excess of Mg2+. Calcium is a macronutrient. Its total content in the body is 1,4%. Calcium is found in every cell of the human body. The bulk of calcium is found in bone and dental tissues. On average, an adult should consume 1 g of calcium per day, although the need for calcium is only 0,5 g. Calcium administered with food is only 50% absorbed in the intestines. Relatively poor absorption is a consequence of the formation in the gastrointestinal tract of sparingly soluble calcium phosphate Ca3(PO4)2 and calcium salts of fatty acids. In the body, the concentration of Ca ions is regulated by hormones. In the bones and teeth of an adult, about 1 kg of calcium is in the form of an insoluble crystalline mineral - hydroxyapatite Ca10(RO4)6(HE)2 , the formation of which occurs during the interaction of Ca ions with phosphation. In the blood and lymph, calcium is found both in an ionized and non-ionized state - in compounds with proteins, carbohydrates, etc. The blood coagulation mechanism consists of a number of stages, depending on the presence of ionized Ca. Ca ions are involved in the transmission of nerve impulses, muscle contraction, regulation of the heart muscle. The concentration of Ca ions inside and outside the cell, respectively, is 106 and (2,25-2,8) 103 mol/l. Since calcium is practically not used inside the cell, it acts as a building material in the body - in bones, teeth. The skeleton is the body's main store of calcium. 39. Biological role of d-elements of the VIB-group. Their use in medicine Chromium is found in plant and animal organisms. The body of an adult contains approximately 6 g of Cr (0,1%). Chromium metal is non-toxic, while Cr(III) and Cr(VI) compounds are hazardous to health. They cause skin irritation, which leads to dermatitis. There is an assumption that derivatives of chromium (VI) have carcinogenic properties. 0,25-0,3 g of potassium dichromate cause death. Chromium (VI) compounds are used as fungicides (pickling agents, fungus - "mushroom", caldere - "kill"). Chromium (III) compounds have a beneficial effect on plant growth. Molybdenum belongs to the "metals of life", being one of the most important bioelements. Its special position was noted 20-25 years ago by F. Krin and L. Oril. These scientists put forward the idea that the emergence of life on Earth did not occur by evolution, but that it was brought by an unknown civilization from space from molybdenum stars, where life existed long before us. In biochemical processes, molybdenum is involved in the oxidation states of VI and VI. In these states, it creates stable oxo forms. Molybdenum forms stable oxo complexes and, apparently, therefore, it is part of the enzymes that ensure the transfer of oxo groups. Mo (VI) predominates in the blood; if the ligand is oxygen, then stable isopolymolybdations are formed. Excessive content of molybdenum in food disrupts Ca metabolism2+ and RO4 , causing a decrease in bone strength - osteoporosis. Perhaps binding into phosphomolybdenum complexes occurs. Such complexes can be considered as acid residues of heteropolymolybdic acids. With calcium, these residues form insoluble crystals. It is possible that these crystals initiate the deposition of uric acid salts and cause gout. Gout deforms the joints, justifying its literal translation - "foot trap". In addition to oxygen complexes, molybdenum forms halide (Hal), thiocyanate (NCS), and cyanide (CN) complexes. Molybdenum is a constituent of various enzymes. In the human body, these include aldehyde hydroxydases, xanthine dehydrogenases, and xanthine oxidases. The molecular weight of xanthine oxidase (COX) is 250 a.u. e. m. This is a molybdenum-containing enzyme of mammals. It can catalyze the oxidation of xanthine and other purines, as well as aldehydes. The conversion of hypoxanthine and xanthine to uric acid is catalyzed by xanthine oxidase. It is assumed that during the catalytic process, molybdenum forms a bond with nitrogen and oxygen of xanthine. Molybdenum is the most important microelement of plants, since biologically active substances with its participation provide mild nitrogen fixation: they convert it into ammonia or nitrogen-containing products. Compared to other industrially important metals, molybdenum has low toxicity. The consumption of molybdenum with food is 0,1 - 0,3 mg / day, but the required daily intake has not been established. Molybdenum deficiency causes a decrease in xanthine oxidase activity in tissues. Excessive content of molybdenum causes osteoporosis. Tungsten is a trace element. Its role in the body is not well understood. The anionic form of tungsten is easily absorbed in the gastrointestinal tract. Metal tungsten and its cationic forms are not absorbed in the body. There is no information about tungsten homeostasis in mammals. 40. Biological role of manganese compounds. Their use in medicine Of the elements of group VIIB, only manganese is a biogenic element and one of the ten "metals of life" necessary for the normal course of processes in living organisms. The body of an adult contains 12 mg. Manganese is concentrated in the bones (43%), the rest - in soft tissues, including the brain. In the body, manganese forms metal complexes with proteins, nucleic acids, ATP, ADP, individual amino acids. Contain manganese metalloenzymes arginase, cholinesterase, phosphoglucomutase, pyruvate carboxylase. The binding of ammonia - a toxic product of the transformation of amino acids in the body of mammals - is carried out through the amino acid arginine. Arginase is an enzyme that catalyzes the hydrolysis of arginine in the liver. As a result, arginine is broken down into urea and the cyclic amino acid ornithine. Urea is a non-toxic, water-soluble substance. It is carried by the blood stream to the kidneys and excreted in the urine. The atomic radius of manganese is 128 pm. This explains the fact that manganese can replace magnesium (atomic radius 160 pm) in its combination with ATP, significantly affecting the energy transfer in the body. Mg and Mn ions also carry out the activation of enzymes - nucleases. These enzymes catalyze the hydrolysis of DNA and RNA nucleic acids in the duodenum. As a result, these biopolymers are split into monomeric units - nucleotides. In particular, such a nuclease is deoxyribonuclease, which catalyzes DNA hydrolysis only in the presence of Mg2+ or Mn2+. Manganese can also be part of inorganic compounds in the body. This, for example, is a poorly soluble manganese magnesium pyrophosphate MnMgP2O7. Crystals of this salt are localized on the inner surface of the vesicle membrane. Almost the same value of the atomic radius of manganese and iron explains the ability of manganese to replace iron in the erythrocyte porphyrin complex. For the same reason, manganese can also replace zinc in zinc-dependent enzymes, thus changing their catalytic properties. Potassium permanganate KMnO4 - the most famous manganese compound used in medicine. Use aqueous solutions containing KMnO4 0,01-5%. A 5% solution is used as a hemostatic agent. Solutions of potassium permanganate have antiseptic properties, which are determined by its high oxidizing power. Of the other manganese compounds, manganese (II) sulfate and manganese (II) chloride should be noted, which are used in the treatment of anemia. There is no data on the presence of technetium in living organisms. However, technetium compounds with bisphosphonates are used for the radioisotope diagnostic method. 41. The biological role of iron compounds. Hemoglobin Iron is a biogenic element found in the tissues of animals and plants. The total mass of iron in the body of an adult is approximately 5 g, which is 0,007%. Metallic iron is low toxic, and Fe (II), Fe (III) and Fe (VI) compounds in large quantities are hazardous to health. Myoglobin, cytochromes, catalase provide cellular respiration. All these proteins consist of their own protein parts and active centers associated with them. The active center is a macrocyclic complex compound - heme. The compound porphyrin acts as a macrocyclic ligand. Donor nitrogen atoms are located at the corners of a square, in the center of which there is an Fe ion. In general, the complex has an octahedral configuration. The fifth orbital through the nitrogen of the amino acid (histidine) is used to bind the heme to the protein. Hemoglobin consists of 4 protein molecules (subunits) that form a single macromolecular aggregate. Each subunit is similar in structure to the myoglobin molecule. Thus, hemoglobin can simultaneously bind four molecules of O2 , and myoglobin - 1. There are also several non-heme iron-containing protein complexes in tissues. These are, for example, enzymes - oxidases, as well as proteins - storage (depot) and carriers of iron. Excess iron is transported in the blood by the protein transferrin and accumulates in the form of ferritin protein in various tissues and organs, especially in the liver, spleen, and bone marrow. Ferritin consists of 24 protein molecules (subunits) that form a sphere with a diameter of 12-14 nm. Each subunit contains a cavity 7 nm in diameter containing up to 4500 iron atoms. Thus, each ferritin aggregate can store approximately 100 iron atoms, providing numerous metabolic reactions involving this element. Based on the laws of chemical equilibrium, it is not difficult to understand the functioning of hemoglobin as an oxygen carrier from the lungs to the tissues. Hemoglobin without oxygen (deoxyhemoglobin) is a weak acid and its chemical formula can be represented as HHb+. The addition of oxygen is accompanied by the elimination of a proton and oxyhemoglobin HbO is formed.2 . In this case, there is an equilibrium: HHb+ + O2 → HbO2 + N+. When oxygen-poor venous blood enters the lungs, where the partial pressure of oxygen is high (up to 20 kPa), its solubility increases according to Henry's law. This leads, in accordance with Le Chatelier's principle, to a shift in equilibrium to the right and the formation of oxyhemoglobin. An additional shift of equilibrium to the right is due to the fact that in the lungs the pH value is increased (up to 7,5). As a result, in the lungs, deoxyhemoglobin is almost completely (up to 97%) saturated with oxygen and passes into oxyhemoglobin. In capillaries penetrating peripheral tissues, the partial pressure of oxygen decreases to 5 kPa, and the pH value decreases to 7,2. As a result, the equilibrium shifts to the left. In the blood flowing from the periphery, hemoglobin is saturated with oxygen only by 65%. 42. The biological role of iron compounds. CO carbon monoxide. The metal-complex properties of heme-containing proteins are manifested under the action of such toxic substances as CO (carbon monoxide) and MCN (cyanides - salts of hydrocyanic acid). The most important from a physiological point of view are iron-containing proteins: hemoglobin, myoglobin, cytochromes, peroxidases, catalase. Hemoglobin - the main component of red blood cells, provides external respiration, being a carrier of oxygen from the lungs to the tissues. Iron Fe and cobalt Co are essential trace elements of living organisms. Carbon monoxide CO is one of the products of incomplete combustion of fuel. Significant amounts of this gas are emitted during the operation of boilers, internal combustion engines, and smoking. When CO is inhaled with air in the lungs in parallel with oxyhemoglobin HbO2 a metal complex compound is formed - carbonyl hemoglobin HbCO. The stability constant of HbCO is about 200 times that of HbO2 . Therefore, even small amounts of CO "intercept" a significant proportion of deoxyhemoglobin, as a result, the supply of oxygen to the organs decreases. There are signs of hypoxia - oxygen deficiency. Nerve tissue is the first to be affected. To detoxify (eliminate the toxic effect) of carbon monoxide, in many cases it is enough to stop its supply and increase oxygen ventilation - take the victim to fresh air. In this case, Le Chatelier's principle works again - the equilibrium shifts towards the formation of oxyhemoglobin. At high concentrations, carbon monoxide blocks the heme-containing proteins of cellular respiration, and it is difficult to avoid a lethal outcome. The mechanism of action of cyanides is similar, but their toxicity is higher than that of CO. The entry into the blood of even very small amounts of these substances leads to respiratory arrest and death. The high toxicity of cyanides is explained by the high strength of the Fe-CN- bond, which determines the greater stability of cyanide hemoglobin. Oxygen respiration leads to the formation of hydrogen peroxide H2O2 . This substance has a high oxidizing power. When it interacts with bioorganic compounds of cells, radicals are formed - very active molecular particles with unsaturated valence, and peroxide oxidation is initiated. Under the action of radicals, the most important components of the cell - membranes and DNA - are destroyed. In the course of biological evolution, nature has developed a special protein - the enzyme catalase, which destroys hydrogen peroxide. This limits the excess accumulation of this substance, and prevents the destruction of the cell. The action of catalase (CatFe2+ ) can be represented as a catalytic cycle of two successive reactions: catfe2+ + N2O2 - Cat Fe2+ × H2O2 , catfe2+ × H2O2 + N2O2 → CatFe2+ + 2Н2O2 + O2 . As a result, 2 hydrogen peroxide molecules are destroyed, and the CatFe2+ biocatalyst molecule is released and can enter the next catalytic cycle. This process is very fast. Within a second, 1 catalase molecule can carry out up to 20 cycles. 43. The biological role of iron and cobalt compounds With a lack of iron in the body, a disease can develop - iron deficiency anemia (anemia). There is tissue oxygen deficiency associated with a lack of iron for the synthesis of hemoglobin. As a result, oxygen delivery to peripheral organs decreases, and, accordingly, the level of cellular respiration decreases, and metabolism slows down. The introduction of iron (II) chloride or iron (II) sulfate as drugs reduces the severity of the disease. For the same purposes, a fine powder of metallic iron (reduced iron, up to 1 g per dose) is used, which is easily soluble in hydrochloric acid of gastric juice. Therefore, the action of this drug is similar to that of iron (II) chloride. However, drugs that are bioinorganic complexes of iron with sugars, nicotinamide and other organic substances are more effective. Such complexes are well absorbed into the blood, which is the reason for their pharmacological effectiveness. It is interesting to note that from ancient times to the present, the so-called iron wine is used to treat iron deficiency anemia - a drink that is obtained by infusing grape wine on iron filings. Obviously, iron dissolves in wine (acidic environment) and forms complexes with natural organic substances, which are contained in it in large quantities. It is clear that the mechanism of action of the ancient drink is approximately the same as that of modern drugs. Like iron, cobalt is also one of the most important biogenic elements. The total mass of cobalt in the body of an adult is approximately 1,2 mg, which is less than 10%. About 100 mg of this mass is in the form of cyanocobalamin (fat-soluble vitamin B12 ) and its analogues. This substance, like heme, is a macrocyclic complex compound. A tetradentate compound, porphin, acts as a macrocyclic ligand. R is a complex organic substituent. In analogs of cyanocobalamin, various organic substituents act instead of the CN anion. The most important role of vitamin B12 plays in the development and formation of red blood cells (erythropoiesis). Vitamin B deficiency12 (receipt of less than 3 mcg per day) leads to a serious illness - pernicious anemia (anemia). It has been established that analogues of cyanocobalamin are activators - cofactors of various enzymes involved in erythropoiesis. The lack of cofactors is manifested in the deficiency of hemoglobin and erythrocytes. Plants and animals cannot synthesize vitamin B12. It is produced only by certain types of bacteria. These bacteria are present in the human gastrointestinal tract. They synthesize enough vitamin B12. Pernicious anemia is associated with impaired absorption of this vitamin into the blood. Therefore, taking pills is ineffective. An injection of vitamin (100-200 mcg for 2 days) into the blood significantly improves the patient's condition with malignant anemia. 44. The role of the d-elements of the IB-group. The use of their compounds in medicine Copper Cu is an essential microelement of living organisms. Silver Ag and gold Au are impurity trace elements. Their compounds are used in medicine. Copper is a biogenic element found in the tissues of animals and plants. The total mass of copper in the body of an adult is approximately 100 mg, which is about 0,0001%. Approximately 30% of this amount is found in the muscles. The liver and brain are also rich in copper. Metallic copper and its compounds are toxic. The most important from a physiological point of view are copper-containing proteins - cytochrome oxidase and superoxide dismutase. Cytochrome oxidase is one of the components of the respiratory chain localized in mitochondrial membranes. Provides cellular respiration by reducing oxygen to water at the end of the respiratory chain. The body needs 2,5-5,0 mg of copper daily. With a lack of copper in the body, a disease can develop - copper deficiency anemia. Copper is necessary for the absorption of iron, in particular, in the synthesis of cytochrome oxidase, which contains both iron and copper. With copper deficiency, the normal development of connective tissues and blood vessels is disturbed. Poisoning is usually associated with an accidental overdose of insecticides, inhalation of metal powder, ingestion of copper salt solutions. Drinks stored in copper vessels without a protective coating of the walls are of great danger. As an external agent, a 0,25% aqueous solution of copper sulfate CuSO is used.4 with inflammation of the mucous membranes and conjunctivitis. Small doses of this drug can be taken with food to increase erythropoiesis in anemia. Silver and gold In the body of an adult, about 1 mg of silver, i.e., approximately 10% (1 part per million), and up to 10 mg of gold, i.e., approximately 10% (10 parts per million), are found. The antiseptic properties of soluble silver salts have been known since ancient times. Priests have known for a long time that water ("holy"), when stored in silver vessels, does not deteriorate for a long time, that is, it is not subject to microbial contamination. Currently, this property of "silver" water is used by sailors on long-distance voyages. Strong toxic manifestations in an adult are observed when 7 g of AgNO is ingested.3. In medicine, drugs such as crystalline silver nitrate AgNO03 (lapis) and its aqueous solutions have long been used. Colloidal metallic silver preparations protargol (8% Ag) and collargol (70% Ag) have long been known, which are fine powders with a metallic sheen. Each particle of such powders is a crystal of reduced metallic silver less than 1 μm in size with a protein coat of albumin (protargol) or collagen (collargol). The protein shell protects the silver crystals from sticking together and ensures their transition into the aqueous medium (solubilizes). Silver preparations are used as anti-inflammatory, antiseptic and astringent agents. Gold preparations are also used as effective anti-inflammatory drugs. The best known are krizanol with a 30% content of the noble metal, and colloidal gold. 45. Biological role of d-elements of group IIB. The use of their compounds in medicine Zinc Zn, cadmium Cd, mercury Hg are trace elements. The body of an adult contains 1,8 g of Zn, 50 mg of Cd, 13 mg of Hg. Cadmium and mercury are impurity elements. About 70% of mercury is concentrated in adipose and muscle tissues. Cadmium is localized by 30% in the kidneys, the rest - in the liver, lungs, pancreas. Zinc is an essential element for all plants and animals. In the body of an adult, most of the zinc in the muscles (65%) and bones (20%). The rest of the amount falls on the blood plasma, liver, erythrocytes. The highest concentration of zinc in the prostate gland. Zinc does not exhibit variable valency. Apparently, therefore, its biocomplexes take part in many biochemical hydrolysis reactions that occur without electron transfer. The Zn ion is part of more than 40 metalloenzymes that catalyze the hydrolysis of esters and proteins. One of the most studied is the bioinorganic zinc complex - the enzyme carbonic anhydrase (Mg = 30), consisting of about 000 amino acid residues. Zinc is not part of dipeptidases - enzymes that catalyze the hydrolysis of dipeptides (substances consisting of 2 amino acids). Zinc forms a bio-inorganic complex with insulin, a hormone that regulates blood sugar. The human need for zinc is fully satisfied by food products: meat, dairy, eggs. With a lack of zinc in plants, protein and carbohydrate metabolism is disturbed, the synthesis of chlorophyll and vitamins is inhibited. Zinc deficiency is eliminated by using zinc-containing fertilizers. The toxicity of group IIB compounds increases from zinc to mercury. Water-soluble compounds irritate the skin and cause poisoning if ingested. The metals themselves are also toxic - when inhaling zinc vapor (the air of zinc production), a "metal" fever appears. Mercury vapor poisoning in the Middle Ages was called the "mad hatter's disease". The content of mercury in food (in marine, as in Japan) leads to minomata disease. Mercury toxicity is associated with agglutination (sticking together) of red blood cells, inhibition of enzymes. For example, sublimate causes a change in size, osmotic fragility and a decrease in the deformability of red blood cells, which is necessary for their movement through the capillaries. The toxicity of cadmium is related to its affinity for nucleic acids. As a result of its attachment to DNA, its functioning is disrupted. Chronic cadmium and mercury toxicity can impair bone mineralization. Toxic elements can replace calcium. This leads to the formation of apatite with an imperfect structure due to the distortion of the parameters of the crystalline component of the bone tissue. As a result, bone strength decreases. 46. Toxic properties of compounds of group IIB (Zn, Cd, Hg) Compounds Zn, Cd, Hg can cause a violation of protein metabolism, which manifests itself in the release of plasma proteins through the kidneys (in proteinuria). The toxic effect of group IIB compounds on the body is also caused by the fact that these metal ions interact with the sulfhydryl SH groups of proteins, enzymes, and amino acids. When metal ions interact with SH groups, weakly dissociating and, as a rule, insoluble compounds are formed. Therefore, the blocking of sulfhydryl groups leads to the suppression of enzyme activity and protein folding. Divalent metal ions simultaneously block two SH groups. In reactions of this type, metal ions act as an acceptor, and sulfur acts as an electron donor. The most pronounced chemical affinity for SH groups in mercury. Obviously, this is due to the fact that the complexing properties of mercury are higher and it forms stronger bonds with sulfur. SH groups are part of more than 100 enzymes, the activity of which can be suppressed due to the blocking of these groups. Therefore, it is obvious how important it is to know the mechanism of blocking and methods of treatment for poisoning the body with metals. It is known that the toxic properties of elements depend on the chemical form in which they enter the body. The most toxic forms are those that dissolve in lipids and easily penetrate through the membrane into the cell. The literature describes a case of mass mercury poisoning in Japan. Inorganic mercury compounds were converted into methylmercury under the action of microbial enzymes. Methylmercury accumulated in fish and then entered the human body with food. Gradually concentrating, methylmercury causes irreversible destruction in the body and death. The use of zinc and mercury compounds in medicine is based on their astringent, cauterizing and antiseptic effects. As eye drops, a 0,25% aqueous solution of zinc sulfate ZnSO is used.4. In dentistry, zinc chloride is used to cauterize papillomas, to treat inflamed mucous membranes. Zinc oxide ZnO is also used. Mercury (II) chloride (mercuric chloride) is very toxic, and its aqueous solutions at high dilutions (1: 1000) are used for disinfection. For the treatment of skin and sexually transmitted diseases, ointments containing mercury oxide (II) HgO and mercury sulfide (II) HgS are used. Mercury(I) chloride (calomel) is poorly soluble in water and therefore slightly toxic. This salt is used in veterinary medicine as a laxative. Mercury under normal conditions is a liquid metal that can dissolve other metals. In this case, hard alloys - amalgams - are formed. In dentistry, silver and cadmium amalgams have long been used for filling teeth. They are chemically inert, soften easily when heated, and are therefore easy to form. Sources of ultraviolet light - mercury-quartz lamps for medical purposes - contain gaseous mercury (vapours). When irradiated with light from these lamps in hospital rooms, microorganisms contained in the air are destroyed. With the help of ultraviolet rays, various skin diseases are treated. Thus, according to the nature of functioning and impact on the body, metals of the IIB group can be divided into the vital element Zn and toxic impurity elements Cd and Hg. 47. Biological role of p-elements of group IIIA. The use of their compounds in medicine Boron is an impurity microelement, its mass fraction in the human body is 105%. Boron is concentrated mainly in the lungs (0,34 mg), thyroid gland (0,30 mg), spleen (0,26 mg), liver, brain (0,22 mg), kidneys, heart muscle (0,21 mg) . The biological effect of boron has not yet been sufficiently studied. Boron is known to be present in teeth and bones, apparently in the form of sparingly soluble salts of boric acid with metal cations. Excess boron is harmful to the human body. There is evidence that an excess of boron inhibits amylases, proteinases, and reduces the activity of adrenaline. According to the content in the human body (105%), aluminum belongs to impurity microelements. Aluminum is concentrated mainly in the blood serum, lungs, liver, bones, kidneys, nails, hair, enters the structure of the nerve sheaths of the human brain. The daily intake of aluminum by a human is 47 mg. Aluminum affects the development of epithelial and connective tissues, the regeneration of bone tissue, affects the exchange of phosphorus. Aluminum has an effect on enzymatic processes. An excess of aluminum in the body inhibits the synthesis of hemoglobin, because due to its rather high complexing ability, aluminum blocks the active centers of enzymes involved in hematopoiesis. There is evidence that aluminum can catalyze the transamination reaction. Gallium is an impurity trace element (the content in the human body is 10−6-10−5%). The biological role of gallium in living organisms is almost not clear. Thallium is a highly toxic element. The T1 ion tends, like Ag+, to form strong compounds with sulfur-containing ligands. As a result, it is very toxic, as it inhibits the activity of enzymes containing thio groups - SH. Even very small amounts of T1 + compounds, when ingested, cause hair loss. Due to the proximity of the radii K+ and T1+ they have similar properties and are able to replace each other in enzymes. Ions T1 and K are synergists. This explains the fact that the enzymes pyruvate kinase and diol dehydratase are activated not only by K ions, but also by T1 ions (the T1 ion replaces the K ion in the catalytic center of enzymes). The synergism of thallium and potassium is also manifested in the fact that, like K ions, T1 ions accumulate in erythrocytes. As an antidote for poisoning with T1 ions, a sulfur-containing ligand, the amino acid cystine, is used. In conclusion, it should be noted that the biological role of group IIIA p-elements has not been sufficiently studied. It is now known that boron and gallium interact in plants with polyphenols, inhibitors of their development, reducing the toxicity of the latter. The undoubted role of aluminum in the construction of epithelial and connective tissues and, in addition, its participation in enzymatic processes, both as an activator and as an inhibitor, has also been established. The T1 ion has the ability to inhibit many sulfur-containing enzymes. The biological activity of group IIIA elements is mainly related to their ability to form complex compounds with oxygen-containing ligands and insoluble phosphates. 48. Biological role of p-elements of the IVA group. The use of their compounds in medicine According to the content in the human body (21,15%), carbon belongs to macronutrients. It is a part of all tissues and cells in the form of proteins, fats, carbohydrates, vitamins, hormones. From a biological point of view, carbon is the number 1 organogen. According to the content in the human body (103% ־), silicon belongs to impurity microelements. Most silicon in the liver, adrenal glands, hair, lens. Since natural silicon dioxide is poorly soluble in water, it enters the human body not so much through the digestive tract as by air through the lungs in the form of dusty SiO2. With a violation of silicon metabolism, the occurrence of hypertension, rheumatism, ulcers, anemia is associated. In medical practice, silicon carbide (IV) SiC is used - carborundum for grinding fillings and plastic prostheses. Silicon dioxide SiO2 part of silicate cements. It should be noted that dust consisting of particles of coal, silicon dioxide and aluminum, when systematically exposed to the lungs, causes a disease - pneumoconiosis. Under the action of coal dust, it is anthracosis, an occupational disease of miners. Inhalation of dust containing S1O2 , silicosis occurs, under the action of aluminum dust - aluminosis. According to the content in the human body (10−6-10−5%) germanium is a microelement. The biological role has not been fully elucidated. Germanium compounds enhance the processes of hematopoiesis in the bone marrow. It is also known that germanium compounds have low toxicity. According to the content in the human body (104 %) tin refers to trace elements. Tin enters the human body with acidic foods preserved in tin cans coated with a layer of tin. In an acidic environment, tin dissolves and enters the blood in the form of a salt, exhibiting a toxic effect. However, in experiments on rats, it was found that tin in small amounts has a stimulating effect on the growth of rats. This gives reason to assume its necessity for humans. Undoubtedly, the elucidation of the biological role of this microelement requires further study. In medical practice, various materials are used, in particular filling materials containing tin. So, tin is part of the silver amalgam (28%) for the manufacture of fillings. Lead and its compounds, especially organic ones, are highly toxic. Lead compounds affect protein synthesis, cell energy balance and its genetic apparatus. Many factors speak in favor of a denaturation mechanism. It has been established that lead is one of the elements whose presence in food affects the development of caries. With food, water, atmospheric air, a person daily absorbs up to 100 micrograms of lead. Lead is deposited mainly in the skeleton (up to 90%) in the form of sparingly soluble phosphate. The mass fraction of lead in the human body is 106%־. A daily intake of 0,2-2 mg of lead is considered safe for humans. In medical practice, lead acetate (lotions) and lead (II) oxide PbO (part of the simple lead patch) have found application as external astringent antiseptics. 49. Biological role of p-elements of the VA group. The use of their compounds in medicine (nitrogen, phosphorus) Nitrogen content in the human body (3,1%) refers to macronutrients. If we take into account only the mass of dry matter of the body (without water), then the nitrogen content in the cells is 8-10%. This element is an integral part of amino acids, proteins, vitamins, hormones. Nitrogen forms polar bonds with hydrogen and carbon atoms in biomolecules. In many bioinorganic complexes (metalloenzymes), nitrogen atoms bind the inorganic and organic parts of the molecule by the donor-acceptor mechanism. Together with oxygen and carbon, nitrogen forms vital compounds - amino acids containing both an amino group with basic properties and a carboxyl group (-COOH) with acidic properties. The amino group also performs a very important function in nucleic acid molecules. The physiological significance of nitrogen-containing bioligands - porphyrins, such as hemoglobin, is enormous. The nitrogen cycle takes place in the biosphere. The nitrogen cycle is vital to agriculture. It is necessary to note one more biologically important property of nitrogen - its solubility in water is almost the same as that of oxygen. The presence of excess nitrogen in the blood can be the cause of the development of decompression sickness. With the rapid ascent of divers, a sharp drop in pressure occurs, the solubility of nitrogen in the blood decreases accordingly (Henry's law), and bubbles of elemental nitrogen leaving the blood clog small vessels, which can lead to paralysis and death. According to the content in the human body (0,95%), phosphorus belongs to macronutrients. Phosphorus is an organogen element and plays an extremely important role in metabolism. In the form of phosphate, phosphorus is an essential component of intracellular ATP. It is part of proteins (0,5-0,6%), nucleic acids, nucleotides and other biologically active compounds. Phosphorus is the basis of the skeleton of animals and humans (calcium orthophosphate, hydroxyapatite), teeth (hydroxyapatite, fluorapatite). Many biosynthetic reactions are carried out due to the transfer of phosphate groups from a high-energy acceptor to a low-energy one. The phosphate buffer system is one of the main buffer systems in the blood. Living organisms cannot do without phosphorus. The importance of phosphorus lies in the fact that sugars and fatty acids cannot be used by cells as energy sources without prior phosphorylation. The exchange of phosphorus in the body is closely related to the exchange of calcium. This is confirmed by a decrease in the amount of inorganic phosphorus with an increase in the content of calcium in the blood (antagonism). The daily human need for phosphorus is 1,3 g. Phosphorus is so widespread in food products that cases of its apparent deficiency (phosphate starvation) are practically unknown. However, not all phosphorus contained in food products can be absorbed, since its absorption depends on many factors: pH, the ratio between calcium and phosphorus in food, the presence of fatty acids in food, but first of all, on the content of vitamin D. A number of phosphorus compounds are used as medicines. It should be noted that organophosphorus compounds containing the C-P bond are strong nerve poisons and are part of chemical warfare agents. 50. Biological role of p-elements of the VA-group (arsenic, antimony, bismuth). Their use in medicine According to the content in the human body, arsenic belongs to trace elements. It concentrates in the liver, kidneys, spleen, lungs, bones, hair. Most arsenic is found in brain tissue and muscles. Arsenic accumulates in the bones and hair and is not completely removed from them for several years. This feature is used in forensic examination to clarify the question of whether there has been poisoning with arsenic compounds. The determination of arsenic in biological material is carried out in a simple device according to the Marsh reaction: zinc and hydrochloric acid are added to the biological object. The hydrogen released during the reaction reduces any arsenic compound to arsine. If the liberated hydrogen contains an admixture of arsine, then AsH decomposes when the gas mixture is heated3 : 2AsH3 = 2As° + 3H2. and on the walls of the tube for gas evolution a black shiny coating of arsenic is formed - an "arsenic mirror". The Marsh reaction is very sensitive and can detect 7-107 g arsenic. In relatively large doses, arsenic compounds are very toxic. As already mentioned, the toxic effect of arsenic compounds is due to the blocking of sulfhydryl groups of enzymes and other biologically active substances. According to the content in the human body (10%), antimony and bismuth are microelements. According to the classification of V. V. Kovalsky, antimony and bismuth belong to the group of microelements that are constantly found in living organisms, but whose physiological and biochemical role is practically not clear. The physiological role of antimony is obviously similar to that of arsenic. Ions of arsenic As and antimony Sb and, to a lesser extent, bismuth Bi are synergists. Thus, it is known that in biogeochemical provinces with an excess of arsenic in organisms, the content of not only arsenic, but also antimony increases. At the same time, both elements accumulate in the thyroid gland of the inhabitants, inhibit its function and cause endemic goiter. The synergism of arsenic and antimony is associated with their ability to form compounds with sulfur-containing ligands. Bismuth, on the other hand, is more inclined to bind to ligands containing amino groups. Thus, the ingestion of soluble bismuth compounds into the body leads to inhibition of the enzymes amino and carboxypolypeptidase. Ingestion of water-soluble antimony compounds, such as stibine SbH3, has a toxic effect similar to arsenic compounds. Bismuth compounds are also toxic when injected. For example, for dogs, the lethal dose is 6 mg/kg body weight. However, when most antimony and bismuth compounds enter the digestive tract, they practically do not have a toxic effect. The weak toxicity of these compounds is due to the fact that Sb (III), Bi (III) salts in the digestive tract undergo hydrolysis with the formation of poorly soluble products that are not absorbed into the walls of the gastrointestinal tract. This is the basis for the use of drugs of antimony and bismuth, for example, basic bismuth nitrate. 51. Biological role of p-elements of the VIA-group. The use of their compounds in medicine According to the content in the human body (62%), oxygen belongs to macronutrients. It is indispensable and is one of the most important elements that form the basis of living systems, that is, it is an organogen. Oxygen is part of a huge number of molecules, ranging from the simplest to biopolymers. The role of oxygen in vital processes is great, since the oxidation of nutrients (carbohydrates, proteins, fats) with oxygen serves as a source of energy necessary for the functioning of organs and tissues of living organisms. Most redox reactions in the body proceed with the participation of oxygen and its active forms. The phagocytic (protective) functions of the body are also associated with the presence of oxygen, and a decrease in the oxygen content in the body lowers its protective properties. In phagocytes (cells capable of capturing and digesting foreign bodies), oxygen 02 is reduced to superoxide. In medical practice, oxygen is used for inhalation in painful conditions accompanied by oxygen deficiency (hypoxia), diseases of the respiratory tract, cardiovascular system, poisoning with carbon monoxide (II) CO, hydrocyanic acid HCN, as well as diseases with impaired respiratory functions. Widely used in clinical practice is hyperbaric oxygenation - the use of oxygen under high pressure. Allotropic modification of oxygen - ozone O3 as a very strong oxidizing agent is used for disinfection of premises, air disinfection and purification of drinking water. According to the content in the human body (0,16%), sulfur belongs to macronutrients. Like oxygen, it is vital. The daily requirement of an adult for sulfur is about 4-5 g. Sulfur is part of many biomolecules - proteins, amino acids (cystine, cysteine, methionine, etc.), hormones (insulin), vitamins (vitamin B1). A lot of sulfur is found in the carotene of hair, bones, nervous tissue. In living organisms, sulfur, which is part of amino acids, is oxidized. The end products of this process are predominantly sulfates. In addition, thiosulfates, cement sulfur and polythionic acids are formed. According to the content in the body (10−5-10−7%) selenium is a microelement. Some researchers consider it to be a vital element. Selenium comes from food - 55-110 mg per year. Selenium is mainly concentrated in the liver and kidneys. The concentration of selenium in the blood is 0,001-0,004 mmol / l. The connection of selenium with sulfur in living organisms is undoubted. At high doses, selenium primarily accumulates in nails and hair, which are based on sulfur-containing amino acids. The ability of selenium to protect the body from poisoning with mercury Hg and cadmium Cd is also known. Selenium promotes the binding of these toxic metals to other active centers, to those that are not affected by their toxic effect. An interesting fact is the relationship between a high content of selenium in the diet and low mortality from cancer. Selenium is toxic in high doses. The breakdown of selenium compounds in animals leads to the release of highly toxic dimethyl selenium, which has a garlic odor. 52. Biological role of p-elements of group VIIA. The use of their compounds in medicine (fluorine and chlorine) According to the content in the human body, chlorine (0,15%) belongs to macroelements, while the remaining elements of this group are microelements (content - 105 %). Halogens in the form of various compounds are part of the tissues of humans and animals. Chlorine and iodine are irreplaceable elements, while the rest are permanent constituents of tissues. The mass of fluorine in the human body is about 7 mg (~105 %). Fluorine compounds are concentrated in bone tissue, nails, teeth. The composition of the teeth includes about 0,01% fluorine, and most of it falls on the enamel, which is associated with the presence of sparingly soluble fluorapatite in it. The lack of fluoride in the body leads to dental caries. Interest in the biological action of fluorine is associated primarily with the problem of dental diseases, since fluorine protects teeth from caries. The mineral basis of dental tissues (dentin) is hydroxyapatite, chlorapatite and fluorapatite. Very often, not the outer surface of the tooth, covered with a layer of enamel, is destroyed, but the inner parts of the dentin, exposed when the enamel is damaged. There are suggestions that while the enamel is slightly damaged, the introduction of sodium fluoride promotes the formation of fluorapatite, facilitating the remineralization of the damage that has begun. Sodium fluoride NaF is used in medical practice as a topical external agent. The use of NaF is based on the formation of fluorapatite. At the same time, alkalization of the oral cavity environment occurs simultaneously, which contributes to the neutralization of acids produced by bacteria. Not only a lack, but also an excess of fluorine is harmful. When the fluorine content in drinking water is above the maximum allowable rate (1,2 mg/l), tooth enamel becomes brittle, easily destroyed, and other symptoms of chronic fluorine poisoning appear - increased bone fragility, bone deformities, and general exhaustion of the body. The disease that occurs in this case is called fluorosis (fluorosis). The human body contains about 100 g (2790 mmol) of chlorine. Chloridions play an important biological role. They activate some enzymes, create a favorable environment for the action of protolytic enzymes of gastric juice, provide ion fluxes through cell membranes, and participate in maintaining osmotic balance. Chloridion has an optimal radius for penetration through the cell membrane. This explains its joint participation with Na and K ions in the creation of a certain osmotic pressure and the regulation of water-salt metabolism. The daily requirement for sodium chloride is 5-10 g. As already discussed, NaCl is necessary for the production of hydrochloric acid in the stomach. In addition to the important role of hydrochloric acid in the process of digestion, it destroys various pathogenic bacteria (cholera, typhoid). If bacteria enter the stomach with a large amount of water, then due to the dilution of HCl, it does not have an antibacterial effect, and the bacteria survive. This leads to illness in the body. Therefore, during epidemics, raw water is especially dangerous. With an insufficient amount of hydrochloric acid in the stomach, the pH rises and normal digestion is disturbed, which seriously affects human health. With reduced acidity of gastric juice, a dilute solution of hydrochloric acid is used in medical practice. With inflammation of the stomach (gastritis), peptic ulcer, the secretion of gastric juice increases, its acidity increases. 53. Biological role of p-elements of group VIIA. The use of their compounds in medicine (bromine, iodine) The mass of bromine in the human body is about 7 mg. It is localized mainly in the endocrine glands, primarily in the pituitary gland. The biological role of bromine compounds in the normal functioning of the body has not yet been sufficiently elucidated. Bromine compounds inhibit the function of the thyroid gland and increase the activity of the adrenal cortex. When bromidions are introduced into the body, the central nervous system is the most sensitive. Bromidions evenly accumulate in various parts of the brain and act calmingly with increased excitability. They contribute to the restoration of the disturbed balance between the processes of excitation and inhibition. Bromidions are easily absorbed in the gastrointestinal tract. The toxicity of bromidions is low. Due to the slow excretion from the body (within 30-60 days), they can accumulate (cumulate), which leads to the development of chronic poisoning, which is called bromism. If signs of chronic bromine poisoning appear, bromine preparations should be immediately discontinued. In addition, a large amount of sodium chloride is administered (up to 25 g per day) to increase the rate of release of bromidions (Le Chatelier's principle), and a plentiful drink is prescribed. Due to different individual sensitivity, the dosage of bromine preparations varies from 0,05 to 2,0 g. Iodine is one of the essential biogenic elements and its compounds play an important role in metabolic processes. Iodine affects the synthesis of certain proteins, fats, hormones. The human body contains about 25 mg of iodine. Of the total amount of iodine in the body, more than half is in the thyroid gland. Almost all of the iodine contained in this gland is in a bound state (in the form of hormones) and only about 1% of it is in the form of iodidione. The thyroid gland is able to concentrate I-25 times - compared to its content in plasma. The thyroid gland secretes the hormones thyroxine and triiodothyronine. An underactive thyroid gland (hypothyroidism) may be associated with a decrease in its ability to accumulate iodide ions, as well as with a lack of iodine in the diet (endemic goiter). With endemic goiter, iodine preparations are prescribed: (potassium iodide KI or sodium iodide NaI) in doses corresponding to the daily human need for iodine (0,001 g of potassium iodide). In areas where there is an iodine deficiency, NaI or K is added to table salt to prevent endemic goiter! (1-2,5 g per 100 kg). With increased activity of the thyroid gland (hyperthyroidism), due to excessive synthesis of thyroid hormones, an abnormally increased rate of metabolic processes is observed. With the ineffectiveness of these drugs for the treatment of hyperthyroidism, a preparation of radioactive iodine 131 I is used, the radiation of which destroys the follicles of the thyroid gland and thereby reduces the excess synthesis of hormones. All elements of group VIIA are physiologically active, and chlorine and iodine are indispensable for the vital activity of the organism. Fluorine is considered an element necessary for the normal functioning of living organisms. In the body, halogens are interchangeable, with cases of both synergy and antagonism being observed. 54. Aerosols Aerosols are dispersed systems with a gaseous dispersion medium. Depending on the state of aggregation of the dispersed phase, fogs are distinguished - aerosols with a liquid dispersed phase; smoke, dust - aerosols with a solid dispersed phase; smog - aerosols with a mixed dispersed phase. The particle sizes of the dispersed phase of aerosols, in accordance with the classification of disperse systems, range from 107 to 109 м. Like other disperse systems, aerosols are obtained by two methods: condensation and dispersion. condensation method The dispersed phase is obtained from the vapor phase by the physical process of condensation of molecules to particles of colloidal size. Dispersion methods Particles of colloidal sizes are obtained by grinding larger aggregates. Aerosols have the ability to scatter light. The particles of the dispersed phase of aerosols do not have a double electric layer, but the particles of the dispersed phase very often carry an electric charge. The charge arises as a result of friction or due to the adsorption of gas ions. It should be noted that very often aerosol particles (small and large) carry a charge of the opposite sign. The separation of particles by size in large volumes of aerosols along the height can lead to the appearance of an electric field of high intensity. Thus, an electrical discharge occurs in the clouds - lightning. Aerosols are kinetically and aggregatively unstable systems, since there is no double electric layer at the phase boundary. Therefore, aerosols coagulate at a faster rate than lyosols. In medicine, aerosols are used in inhalation therapy, to protect damaged skin, disinfection. Sometimes the formation of aerosols is highly undesirable. Aerosols hazardous to human health are formed in the foundry, ceramic industries, during the extraction and processing of various minerals (ore, coal, asbestos, etc.). Aerosols containing particles of coal cause lung disease - anthracosis, silicon (IV) oxide - silicosis, asbestos - asbestosis. Allergic diseases are caused by aerosols formed by plant pollen, dust generated during the processing of cotton, flax, hemp, etc. Suspensions of bacteria, mold and viruses - microbiological or bacterial aerosols - are one of the ways of transmitting infectious diseases: pulmonary tuberculosis, influenza, acute respiratory diseases. Harmful effects on the human body are produced by aerosols formed during the combustion of fuel, the dispersed phase of which consists of soot, tar, ash, and carcinogenic hydrocarbons. Smogs are especially hazardous to health. Therefore, the fight against dust and pollution of the atmosphere is becoming increasingly important. Air purification from aerosols is achieved by introducing non-waste technologies - trapping particles of the dispersed phase using filters, cyclones (centrifugal dust collectors), and a high-voltage electric field. 55. Emulsions Emulsions are microheterogeneous systems in which the dispersed phase and the dispersion medium are immiscible liquids. The particle sizes of the dispersed phase - liquid droplets - range from 104 to 106 m. Depending on the concentration of the dispersed phase, emulsions are distinguished: diluted, concentrated and highly concentrated. Depending on the nature of the dispersed phase and the dispersion medium, there are: 1) emulsions of non-polar liquid (DF) in polar (DS) - direct emulsions, called emulsions of the first kind or emulsions of the "oil / water" type (O / W); 2) emulsions of a polar liquid (DF) in a non-polar one (DS) - inverse emulsions, called emulsions of the second kind or emulsions of the "water / oil" type (W / O). Here, DF and DS are the dispersed phase and the dispersion medium, respectively, "water" is any polar liquid, "oil" is non-polar. Emulsion type can be set: 1) measurement of electrical conductivity; 2) mixing with an excess of a polar or non-polar liquid; 3) staining with water-soluble or oil-soluble dyes; 4) by wetting and spreading of an emulsion drop on a hydrophobic or hydrophilic surface. Emulsions, like other disperse systems, can be obtained by condensation and dispersion methods. Emulsions as coarse dispersions are kinetically and aggregatively unstable systems. When droplets of the dispersed phase collide, they merge (coalescence). As a result of coalescence, the emulsion separates into two continuous liquid phases. Emulsifiers are used to increase the stability of emulsions. These are surfactants, which, as a result of adsorption at the phase boundary, reduce the value of interfacial tension and form a mechanically strong adsorption film. If the emulsifier is an ionic surfactant, then it imparts an electric charge of the same sign to the droplets of the dispersed phase, and the droplets repel each other. The type of emulsion formed depends on the properties of the emulsifier. The dispersion medium is always the liquid that best dissolves or wets the emulsifier. Salts of higher fatty acids, esters of higher fatty acids and polyhydric alcohols, long-chain amines are used as emulsifiers. Emulsions are widely found in nature. Emulsions are milk, cream, sour cream, butter, egg yolk, milky plant juice, crude oil. Emulsions containing medicinal substances are widely used in medicine: the first kind (O/W) for internal use, the second kind (W/M) for external use. It is known that vegetable and animal fats are better absorbed by the body in an emulsified form (milk). In this case, derivatives of cholic and deoxycholic acids act as emulsifiers. Sometimes there is a need to break the resulting emulsion. The breaking of an emulsion is called demulsification. Demulsification is carried out by raising and lowering the temperature, exposure to an electric field, centrifugation, adding electrolytes and special substances - demulsifiers. Demulsifiers are surfactants with greater surface activity than emulsifiers, but do not have the ability to form a mechanically strong adsorption layer. 56. Colloidal surfactants Colloidal surfactants are substances that, with the same solvent, depending on the conditions, form a true and colloidal solution. As already mentioned, surfactant molecules are amphiphilic. They consist of non-polar and polar groups. Non-polar radicals, for example, hydrocarbon chains, have no affinity for the polar solvent - water, for polar groups it is quite high. There is a hydrophobic (van der Waals) interaction between non-polar groups. With a chain length of approximately 1022 carbon atoms due to hydrophobic interactions of hydrocarbon radicals and the strong interaction of polar groups with water, the association of surfactant molecules occurs and micelles are formed. The minimum concentration of a colloidal surfactant, starting from which micelles form in its solution, is called the critical micelle concentration (CMC). The shape of the resulting micelles depends on the concentration of the solution. At low concentrations of colloidal surfactant, spherical micelles are formed. An increase in the concentration of a colloidal surfactant solution first leads to an increase in their number, and then to a change in shape. At higher concentrations, instead of spherical micelles, cylindrical and lamellar micelles are formed. The CMC value depends on various factors: the nature of the colloidal surfactant, temperature, and the presence of impurities of foreign substances, especially electrolytes. CMC can be determined by the properties of the solution, which depend on the number and size of kinetically active particles, in particular, by changes in osmotic pressure, surface tension, electrical conductivity, and optical characteristics. Since the size of kinetically active particles (ions, molecules, micelles) and their number change during the transition "true solution - colloidal solution", a break point corresponding to CMC appears on the "property - concentration" graph. One of the most important properties of colloidal surfactant solutions, due to which they are widely used in various sectors of the national economy and in medicine, is solubilization. The mechanism of solubilization consists in the dissolution of non-polar substances in the hydrophobic core of micelles. The phenomenon of solubilization is widely used in various sectors of the national economy: in the food industry, in the pharmaceutical industry (to obtain liquid forms of medicinal substances). In the "water - phospholipid" system, shaking and stirring form spherical micelles - liposomes. Phospholipid molecules form a bilayer membrane in liposomes, in which polar groups face water, and non-polar ones face each other. Liposomes can be considered as a model of biological membranes. With their help, it is possible to study the permeability of membranes and the influence of various factors on it for various compounds. Liposomes are widely used for targeted delivery of drugs to certain organs or affected areas. Liposomes can transport drugs into cells. Liposomal membranes are used in immunological studies to study the interaction between antibodies and antigens. Authors: Drozdova M.V., Drozdov
▪ General psychology. Lecture notes ▪ Labor law of the Russian Federation. Crib ▪ Money. Credit. Banks. Lecture notes
Artificial leather for touch emulation
15.04.2024 Petgugu Global cat litter
15.04.2024 The attractiveness of caring men
14.04.2024
▪ section of the site Electrician's Handbook. Article selection ▪ article Study, study and study. Popular expression ▪ article Against which landmark did 300 figures of French culture write a protest? Detailed answer ▪ article Working with a fitter's and assembly tool. Standard instruction on labor protection ▪ article Mouthpiece and cigarette. Focus secret
Home page | Library | Articles | Website map | Site Reviews www.diagram.com.ua |






 Arabic
Arabic Bengali
Bengali Chinese
Chinese English
English French
French German
German Hebrew
Hebrew Hindi
Hindi Italian
Italian Japanese
Japanese Korean
Korean Malay
Malay Polish
Polish Portuguese
Portuguese Spanish
Spanish Turkish
Turkish Ukrainian
Ukrainian Vietnamese
Vietnamese

 See other articles Section
See other articles Section 